calsfoundation@cals.org
Fleas
Fleas are small, wingless, hematophagous (blood-feeding) ectoparasites that belong to the Phylum Arthropoda, Class Insecta, and Order Siphonaptera. There are four recognized suborders—Ceratophyllomorpha, Hystrichopsyllomorpha, Pulicomorpha, and Pygiopsyllomorpha—with about 246 recognized genera and over 2,500 described species within sixteen families. Adult fleas feed on blood of mostly mammals (about ninety-four percent of known species), including dogs, cats, and humans, with the remainder of species parasitizing birds. Fleas are an important component of the worldwide biota. In addition, they can be nuisance biters, and some serve as vectors or intermediate hosts of flea-borne disease agents and parasites. The most recent summary listed twenty-nine species of fleas in Arkansas.
Fleas are most closely related, evolutionarily speaking, to insects in the orders Diptera (true flies) and Mecoptera (scorpionflies). The family Boreidae (Mecoptera—snow scorpionflies) is the sister clade to the Siphonaptera. Fleas first appeared in the early Cretaceous Period (sixty-five to 146 million years ago), most likely as ectoparasites of primitive marsupials and other mammals, before infesting other groups, including birds. A Cenozoic Era fossil of a flea that appears morphologically similar to modern fleas, dated to approximately 20 million years ago, was found in amber.
Many flea species are more or less host specific and do not feed on any other host, though some lack host specificity. For example, members of some families of fleas are exclusive to a single host group, including the families Malacopsyllidae (found only on armadillos), Ischnopsyllidae (found only on bats), and Chimaeropsyllidae (found only on elephant shrews).
Some famous siphonapterists of the past include Nathan “Charles” Rothschild (1877–1923), who created the world’s greatest flea archive, which is in the Rothschild Collection at the Natural History Museum in London, and his daughter, Dame Miriam Louisa Rothschild (1908–2005), also a noted flea authority; G. H. E. (Harry) Hopkins (1898–1973), who co-authored with Miriam Rothschild a series of tomes on the flea collections in London’s Natural History Museum; Irving Fox (1912–?), who published the classic Fleas of Eastern United States in 1940; Henry E. Ewing (1883–1951), who with Clarence Andreson (C. A.) Hubbard published Fleas of North America in 1943; C. A. Hubbard, who published the classic Fleas of Western United States in 1947 and Fleas of California in 1943; Robert Traub (1916–1996), who amassed one of the world’s largest flea collections (now curated at the Carnegie Museum of Natural History in Pittsburgh, Pennsylvania); Cluff E. Hopla (1917–2008) of the University of Oklahoma; Robert E. Lewis (1929–2017), who amassed a large Iowa state flea collection and published extensively on the subject; Franciscus Gerardus Albertus Maria Smit (1920–2000), late custodian of the Rothschild collection of siphonaptera at Tring, Hertfordshire, England; J. L. (Jay) Lancaster Jr. (1923–2016), who provided information on Arkansas fleas; and Nixon Wilson (1930–2011) of the University of Northern Iowa, who served in the Plague Research Unit in Hawaii and as an acarologist in the Bernice P. Bishop Museum in the same state working on fleas and other ectoparasites.
Adult fleas range in size from one to eight millimeters long and are laterally compressed wingless insects; compound eyes are absent, but some have ocelli (eye spots) with a single biconvex lens, while some species lack eyes altogether. The body of a flea is covered with hard plates called sclerites that are covered with many hairs (setae) and short spines directed backward, which also assist in movements on the host. Flea legs end in strong claws that are designed to grasp a host. Their hind legs are longer than the others and are used for jumping. A protein called resilin is associated with the pleural arch of the legs and is capable of releasing ninety-seven percent of its potential energy to execute a jump. Fleas also possess a pygidium, a posterior sensory structure that detects air currents. Some species have a series of thick, sharp spines arranged as combs (ctenidia) including a genal ctenidium on the head and a pronotal ctenidium on the posterior portion of the first thoracic tergite. Body setae are generally arranged in dorso-ventral rows and directed posteriorly. These are used to keep fleas from being easily dislodged by a host’s grooming or scratching. With host-specific species of fleas, the spacing between the rows tends to correlate with the average diameter of hair shafts of a preferred host.
When a flea feeds, its maxillary lobes (laciniae) cut back and forth, and a proboscis (or stylet) pierces the skin and sucks the host’s blood through a median, unpaired epipharynx. This epipharynx is elongate, which allows it to enter a blood vessel. Saliva then enters the wound created by the laciniae but does not enter the blood vessel. Interestingly, adult fleas can live for long periods of time without feeding; however, each species is different, and some may survive without host blood for over a year. A few species can even withstand freezing for months at a time.
Fleas are holometabolous insects that have four life cycle stages: egg, larva, pupa, and imago (adult). Females typically lay eggs on or near a given host. Generally, a few to up to twenty eggs are laid at a time, and over a course of two to six months (depending on the species), perhaps several hundred eggs may be laid. In some species, egg-laying is strongly related to humidity. Eggs usually fall to ground within a few hours of drying. Eggs hatch within a few days but sometimes take three weeks or longer to hatch, depending upon temperature. Maggot-like larvae hatch and ingest detritus (decaying vegetation or host epithelial cells) or dried host blood voided by conspecific adult fleas, usually within a nest. The majority of species have three larval instars, and each instar lasts about 1.5 to two weeks. However, low temperatures can prolong a stage for up to 200 days in some species. Larvae tend to seek out areas with appropriate humidity, within less than one meter from the hatching source. A pupa forms, and much of its case is formed from silk-like material derived from salivary secretions and detritus picked up from the environment. Development in the pupa generally lasts about one week. However, low temperatures can retard pupal development for up to a year.
Fleas are apparently sensitive to odors, and particular species can home in on specific hosts based on these odors. They also typically cue in on temperature and light, especially light/shadow/light scenarios.
Several flea species have medical-veterinary importance. Some species may transmit disease agents such as Francisella tularensis, the causative agent of tularemia; Bartonella henselae, the causative agent of cat scratch disease; Rickettsia felis, the agent of cat flea rickettsiosis; and Rickettsia typhi, the causative agent of murine typhus. Some can also serve as intermediate hosts of protist and helminth parasites, including an avian-associated trypanosome, the double-pored dog tapeworm (Dipylidium caninum), dwarf tapeworm (Hymenolepis nana), rat tapeworm (Hymenolepis diminuta), and a filarial worm of dogs (Dipetalonema reconditum).
In addition to these flea-borne pathogens and parasites, some fleas are nuisance biters. Some pets and humans are hypersensitive to flea bites and develop flea bite allergies that lead to intense itching, scratching, dermatitis, and the possibility of secondary bacterial infections. There is also circumstantial evidence that rodent-associated fleas transmit Bartonella spp. and other bacteria to their hosts. Most notably, the Oriental or tropical rat flea, Xenopsylla cheopis, is a vector of the bacterium that causes bubonic plague, Yersinia pestis.
Some representative species of fleas of the family Pulicidae include the cat flea (Ctenocephalides felis), a nuisance biter of domestic cats and wild felids but is also common on domestic dogs; Hoplopsyllus anomalus, which infects California ground squirrels and rats; Pulex simulans (a canid/coyote flea) found on many mammals in North America; and X. cheopis, which prefers rats but will bite other hosts. The last species is found worldwide and is an efficient vector of Rickettsia typhi, the causative agent of murine typhus. Human cases of murine typhus were recorded in Arkansas in the first half of the twentieth century until intensive domestic rat and flea control operations were implemented throughout the southern United States. Another important member of this family is the human flea, Pulex irritans, a widely distributed ectoparasite mainly of medium-sized and large mammals. It can serve as intermediate host of D. caninum and H. nana, which can also be transmitted to humans, especially children who have close contact with flea-infested cats and dogs. The role of this flea in human-to-human transfer of the plague bacterium is uncertain, but it is thought to be significant in some outbreaks.
The family Ceratophyllidae includes the western chicken flea (Ceratophyllus niger); Oropsylla montana, found on squirrels in the western and central United States; and the Northern rat flea (Nosopsyllus fasciatus) of Europe and North America. An invasive species, Echidnophaga gallinacea (the sticktight flea) infests birds and mammals. It is often found on the heads of chickens, and occasionally on companion animals like dogs and cats that have had with contact with barnyard birds. This small flea is globally widespread in tropical, subtropical, and warm temperate regions, including Arkansas. This flea has elongate, barbed mouthparts that allow it to embed in host tissue for prolonged periods, as reflected by its common name. It sometimes occurs in clusters of specimens on the host head where oral self-grooming cannot be directed against it by the host. It tends to congregate in masses and prefers soft tissues devoid of hair or feathers, especially in avian hosts, around the eyes, on the comb, and around the anus. Inflammation from heavy infections can cause chickens’ eyes to swell shut.
There are other flea families of note from various hosts in Arkansas. An example of a flea from the Family Rhopalopsyllidae is Polygenis gwyni. The hispid cotton rat (Sigmodon hispidus) is the most commonly recorded host of P. gwyni, but they can also be found on the Virginia opossum, Didelphis virginiana. The Family Ctenophthalmidae includes a common flea of shrews, Corrodopsylla hamiltoni. The principal host is the least shrew, Cryptotis parva, which has been recorded mainly in the Midwest but can be found as far south as north-central Texas. It has no known medical-veterinary importance. Fleas of the Family Leptopsyllidae include Odontopsyllus multispinosus, a large flea associated with leporids and their predators in eastern North America. It has no known medical-veterinary importance, but it could be an enzootic vector of F. tularensis. One important species in the Family Ischnopsyllidae is Nycteridopsylla chapini, an ectoparasite of bats, especially the big brown bat (Eptesicus fuscus) in the eastern and midwestern United States. A specific ectoparasite of the raccoon, Procyon lotor, in eastern North America from Maine to North Carolina westward to Ontario and Arkansas is Chaetopsylla lotoris (Family Vermipsyllidae).
Economically, fleas have had a significant negative impact on the economy of pet owners. In the United States alone, approximately $2.8 billion is spent annually on flea-related veterinary bills and another $1.6 billion annually for flea treatment with pet groomers. At total of $4 billion is spent annually for various over-the-counter and prescription flea treatments, and $348 million for flea pest control.
The first attempt at listing the fleas of Arkansas showed twenty-one species. However, those collections (made in 1968) were limited to hosts from northwestern Arkansas. A more recent summary listed twenty-nine species of fleas (within seven families) from the state. That summary included new records of the following fleas: Ctenophthalmus pseudagyrtes from the golden mouse (Ochrotomys nuttalli), hispid cotton rat (Sigmodon hispidus), southern short-tailed shrew (Blarina carolinensis), and eastern mole (Scalopus aquaticus); Doratopsylla blarinae from B. carolinensis and the northern short-tailed shrew (Blarina brevicauda); Orchopeas howardi from the southern flying squirrel (Glaucomys volans), eastern gray squirrel (Sciurus carolinensis), and eastern fox squirrel (Sciurus niger); and Orchopeas leucopus from the Texas mouse (Peromyscus attwateri), white-footed mouse (Peromyscus leucopus), and deer mouse (Peromyscus maniculatus). Although much has been documented recently on Arkansas fleas, additional scrutiny of birds and mammals, including the examination of their nests, is warranted, for the possibility of recording additional fleas in the state.
For additional information:
Baerg, William J. “Ticks and Other Parasites Attacking Northern Cliff Swallows.” Auk 61 (1944): 413–414.
Caster Paul T., Gary A. Heidt, and Karen D. Stone. “Faunal Use of Nest Boxes in the Ouachita Mountains of Central Arkansas.” Southwestern Naturalist 39 (1994): 380–382.
Connior, Matthew B., Lance A. Durden, and Chris T. McAllister. “New Records of Ectoparasites and Other Epifauna from Scalopus aquaticus and Blarina carolinensis in Arkansas.” Journal of the Arkansas Academy of Science 68 (2014): 137–139. Online at http://scholarworks.uark.edu/jaas/vol68/iss1/23/ (accessed December 2, 2017).
Durden, Lance A., and N. Hinkle. Fleas (Siphonaptera). In Medical and Veterinary Entomology, edited by G. R. Mullen and Lance A. Durden. Amsterdam: Elsevier, 2009.
Hopkins, George H. E., and Miriam Rothschild. An Illustrated Catalogue to the Rothschild Collection of Fleas. 6 vols. London: British Museum (Natural History), 1953–81.
Hopla, Cluff E. A Study of the Host Associations and Zoogeography of Pulex. In Fleas: Proceedings of the International Conference on Fleas, edited by Robert Traub and H. Starcke. Rotterdam: A. A. Balkema, 1980.
Hubbard, Clarence A. Fleas of the Western North America: Their Relationship to the Public Health. Ames: Iowa State College Press, 1947.
Krasnov, Boris R. Functional and Evolutionary Ecology of Fleas: A Model for Ecological Parasitology. London: Cambridge University Press, 2008.
Lewis, Robert E. “Resume of the Siphonaptera (Insecta) of the World.” Journal of Medical Entomology 35 (1998): 377–389.
———. “A Taxonomic Review of the North American Genus Orchopeas Jordan, 1933 (Siphonaptera: Ceratophyllidae: Ceratophyllinae).” Journal of Vector Ecology 25 (2000): 164–189.
Lewis, Robert E., and T. D. Galloway. “A Taxonomic Review of the Ceratophyllus Curtis, 1832 of North America (Siphonaptera: Ceratophyllidae: Ceratophyllinae).” Journal of Vector Ecology 26 (2001): 119–161.
Lewis, Robert E., and Nixon Wilson. “A New Species of Nycteridopsylla (Siphonaptera: Ischnopsyllidae) from Southwestern United States, with a Key to the North American Species.” Journal of Medical Entomology 19 (1982): 605–614.
McAllister, Chris T. “First Record of Corrodopsylla hamiltoni (Siphonaptera: Hystricopsyllidae) in Texas.” Southwestern Naturalist 34 (1989): 561–563.
McAllister, Chris T., and Nixon Wilson. “Ctenophthalmus pseudagyrtes (Siphonaptera: Ctenopthalmidae): New to the Flea Fauna of Texas.” Southwestern Naturalist 57 (2012): 345–346.
McAllister, Chris T., Matthew B. Connior, and Lance A Durden. “Ectoparasites of Sciurid Rodents in Arkansas, Including New State Records for Neohaematopinus spp. (Phthiraptera: Anoplura: Polyplacidae).” Journal of the Arkansas Academy of Science 67 (2013):197–199. Online at http://scholarworks.uark.edu/jaas/vol67/iss1/34/ (accessed December 2, 2017).
McAllister, Chris T., Lance A. Durden, Henry W. Robison and Matthew B. Connior. “The Fleas (Arthropoda: Insecta: Siphonaptera) of Arkansas.” Journal of the Arkansas Academy of Science 71 (2017).
McKern, J. A., A. L. Szalanski, J. W. Austin and R. E. Gold. “Genetic Diversity of Field Populations of the Cat Flea, Ctenocephalides felis, and the Human Flea, in the South Central United States.” Journal of Agricultural and Urban Entomology 25 (2008): 259–263.
Mullen, Gary R., and Lance Durden. Medical and Veterinary Entomology. New York: Academic Press, 2009.
Richardson, Dennis J., Lance A. Durden, and Daniel E. Snyder. “Ectoparasites of the Raccoon (Procyon lotor) from North-Central Arkansas.” Journal of the Kansas Entomological Society 67 (1994): 208–212.
Richardson, Dennis J., and A. M. Mangili. “Infection with the Sand Flea Tunga penetrans (Tungiasis) in a Traveller Returning from Cameroon, Africa.” Journal of the Arkansas Academy of Science 70 (2016): 199–206. Online at: http://scholarworks.uark.edu/jaas/vol70/iss1/33/ (accessed December 2, 2017).
Sanderson, M. W. “A Bat Flea New to Arkansas.” Journal of the Kansas Entomological Society 14: (1941): 60.
Schiefer, B. A., and Jack L. Lancaster Jr. “Some Siphonaptera of Arkansas.” Journal of the Kansas Entomological Society 43 (1970): 177–181.
Tumlison, Renn, Matthew B. Connior, Henry W. Robison, Chris T. McAllister, Lance A. Durden, D. Blake Sasse, and David A. Saugey. “Vertebrate Natural History Notes from Arkansas, 2015.” Journal of the Arkansas Academy of Science 69 (2015): 106–115. Online at http://scholarworks.uark.edu/jaas/vol69/iss1/21/ (accessed December 2, 2017).
Chris T. McAllister
Eastern Oklahoma State College
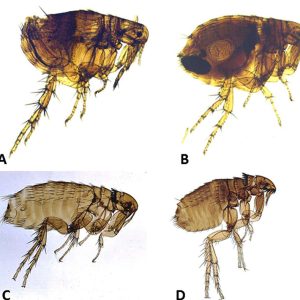 Flea Species
Flea Species 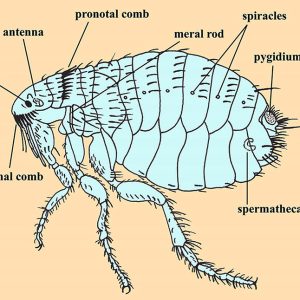 Flea Species
Flea Species 




Comments
No comments on this entry yet.