calsfoundation@cals.org
Leeches
Leeches are segmented worms belonging to the Phylum Annelida, Class Clitellata, Subclass Hirudinida. Leech classification is primarily based on the presence or absence of setae (bristles) and the morphology of the mouth, proboscis (feeding organ), jaws, and suckers. Leeches are thought to have evolved from certain oligochaete worms; however, the systematics and taxonomy of leeches are in need of review. Twenty-two species within five families (Erpobdellidae, Glossiphoniidae, Haemopidae, Hirudinidae, Piscicolidae) have been reported from northern Arkansas, but, as of 2018, there are no summaries of leeches from the southern part of the state.
Leeches are bilaterally symmetrical, with thick muscular bodies. Usually, they are dorsoventrally flattened and segmented. Some leeches are long and worm-like (ranging in size from about seven to 300 mm long when extended), while others are broad or pear-shaped. Most vary considerably in shape both between the elongated and contracted state and between the starved (unfed) and fed condition.
Worldwide, there are nearly 700 species recognized in three orders, of which about 100 are marine and 90 are terrestrial, with the majority being freshwater taxa. These are divided into two major infraclasses: the Euhirudinea or “true” leeches, which have suckers at both ends and lack setae, and the Acanthobdellida, a small Northern Hemisphere infraclass that is ectoparasitic on salmonid fish and lacks an anterior sucker and retains setae. The Euhirudinea are further divided into two orders: Arhynchobdellida and Rhynchobdellida. Worldwide, there are ten families of Euhirudinea, and in North America north of Mexico there are eight families, nineteen genera, and at least eighty-one nominal species of leeches.
Leeches are cosmopolitan invertebrates, and some are found in great abundance. They occur in a wide range of ecosystems including estuarine, marine, moist terrestrial, and freshwater. In these latter systems, leeches are found in ponds, lakes, swamps, streams, and rivers, where they are an integral component of the benthic (deep-water) and occasionally pelagic (open-sea) communities. Leeches are most abundant along shallow vegetated shoreline. Freshwater leeches are important invertebrates of benthic communities in almost every freshwater ecosystem, and some prefer to live in still or slowly flowing waters, but a few species also occur in fast-flowing streams. Many are predators, feeding by swallowing other invertebrates, or are scavengers, with about twenty-five percent of species being non-parasitic. Terrestrial leeches are common on the ground or in low foliage in wet rain forests; they never enter water and cannot swim, although they can survive periods of immersion. In dry climates, some species burrow in the soil, where they can survive for many months even in a total lack of standing water. In these conditions, the body is contracted to be dry and rigid, the suckers are difficult to distinguish, and the body surface is completely dry. However, if placed in a few drops of water, these leeches emerge, fully active.
Although leeches have not been used very often at the species level for bioassessment and monitoring water quality, their presence/absence, habitats, and certain environmental requirements have been documented in ecological investigations. Additional studies on the pollution and water-quality tolerances of leeches are required before the effects of environmental stresses and chemical pollution on this group of invertebrates are understood.
Medicinal leeches have been used for thousands of years. The early Egyptians and Mesopotamians used leech therapy over 3,500 years ago, and leeches are even included in the hieroglyphics painted on the walls of ancient buildings. Blood-letting was common in ancient Greece, India, Persia, and Rome, and in the days of the Mayan and Aztec civilizations.
The early American colonists were big proponents of blood-letting. George Washington probably died as a result of overzealous blood-letting when he was bled four times in two days after developing a severe sore throat. The emperor Napoléon Bonaparte imported nearly six million leeches in one year to treat his soldiers. By the mid-1800s, the demand for leeches was so high that the French imported about 40 million leeches a year for medicinal purposes, and the following decade England imported six million leeches a year from France alone. Johann Friedrich Dieffenbach (1792–1847) regularly and successfully utilized leeches in sophisticated plastic surgery in Berlin in the 1820s and 1830s, well before anesthesia, antisepsis, and antibiotics. There was also an explosion in the use of leeches in Asia and the Middle East.
During the latter half of the nineteenth century, therapeutic excesses began to bring the practice of blood-letting into ill repute. However, the use of leeches never stopped in some parts of the world, especially in Germany and Russia. During the twenty-first century, studies were done in which leeches were tried for certain ailments such as arthritis, and the U.S. Food and Drug Administration gave permission for sale and use of leeches in the United States but limited its use in microsurgeries for re-establishing blood circulation and plastic surgeries only. Leeches are enjoying a revival for known health benefits, including new discoveries based on thorough medical research about positive effects of the substances produced by medicinal leeches that are later induced by leeches into the human body (and/or animals) during the hirudotherapy treatments.
Although native populations of H. medicinalis still occur rarely in some parts of Britain and southern Norway to the southern Urals and probably as far as the Altai Mountains and the countries bordering the northeastern Mediterranean, it is not native to North America. It was introduced into the United States and currently has small, scattered populations in some areas. Major threats to the species are collecting pressure, loss of wetland habitats, the global decline of amphibians, and abandonment of traditional livestock grazing practices. Indeed, drained and agriculturally ameliorated stretches are devoid of European medicinal leeches. Due to the associated projected population decline, the International Union for Conservation of Nature (IUCN) Red List lists this leech as Near Threatened and is likely to move it to a Vulnerable category.
Some famous leech biologists include Donald J. Klemm, William E. Moser, Dennis J. Richardson, Roy T. Sawyer, and Mark E. Siddall. Two of them, Moser and Richardson, have published extensively on the leeches of Arkansas.
Most leeches have thirty-four internal segments, of which there are two preoral and thirty-two postoral segments. Segments are further divided into annuli, and the number of annuli (two to sixteen) varies among leech taxa. Of these thirty-two postoral segments, the first four anterior segments are designated head segments and include an anterior ganglion and sucker; these are followed by twenty-one midbody segments (including twenty-one neuronal ganglia, two reproductive organs, and nine pairs of testes). Finally, the last seven segments are fused to form the animal’s tail sucker, as well as its posterior ganglion. Leeches have two suckers, an anterior (pseudosucker) and a large posterior sucker, and most are without setae. Several are capable of swimming, while others cannot. Leeches have a complete gut, sometimes with diverticulae. Some have eyes and sensory papillae embedded over their body surface.
Most leeches are sanguivorous (feed as bloodsucking parasites) on preferred hosts. However, nearly one-half of leech species prey on small invertebrates (crustaceans, earthworms, gastropods, and insect larvae), which they usually swallow whole. Many feed solely on decomposing bodies and on open wounds of fishes, amphibians, reptiles, waterfowl, and mammals. Arhynchobdellid leeches feed by either swallowing their prey whole or creating a hemorrhage with their jaws and teeth and consuming the pooled blood or invertebrate body fluids. Rhynchobdellid leeches feed by protruding their proboscis into host tissue, which is aided by releasing salivary proteolytic enzymes at the tip and sucking this fluid. The host blood is prevented from clotting by production of a non-enzymatic secretion (anticoagulant) called hirudin or by an enzymatic secretion called hemetin. Some leeches will even take a meal from other sanguivorous leeches, which may die after the attack. Sanguivorous leeches can ingest several times their own weight in blood during a single meal. In these species, symbiotic bacteria aid in the digestion of blood meals by producing enzymes that assist in the breakdown of blood and producing vitamins, whereas non-blood-feeding leeches lack bacterial endosymbionts. After feeding, the leech may fall off the host and retire to a dark spot to digest its meal. Digestion is slow, which enables leeches to survive during very long fasting periods (up to several months). Depending on the species and size, human leech bites may go unnoticed, or they can be fairly painful.
Additional structures of the digestive tract (foregut section) include salivary glands and an esophagus. The salivary glands are typically scattered diffusely in the anterior one-third of the body, but in some taxa (glossiphoniids) they occur in discrete clusters. The salivary glands of sanguivorous species are primarily involved in the mechanics of blood-feeding by the secretions of anticoagulation, vasodilation, spreading factor, and inflammatory response inhibition compounds. In sanguivorous rhynchobdellids, their esophagus is adorned with structures that harbor endosymbiotic microorganisms. These structures are termed mycetomes and may be a glandular esophageal organ, or are bulbous or evaginated sacs. On the other hand, salivary glands of predaceous leeches are primarily involved in the digestion of food.
Leeches have a midgut composed of the crop (expandable food storage compartment), intestine, and rectum. In predatory leeches, the crop may be a simple tube; however, in sanguivorous species, the crop has many branched caeca, which greatly increases the storage space. The posterior end of the crop in both predaceous and sanguivorous species contains the post-caeca, which extends down to the intestine. The intestine is acaecate, except in the Glossiphoniidae where the anterior portion of the intestine contains four pairs of caeca. The size of the anus is typically related to feeding habits. Sanguivorous leeches have a small anus, and predaceous leeches have a larger anus.
Leeches are segmented hermaphrodites, meaning each has both male and female reproductive systems; the male sexual system matures before the female sexual system. The typical life cycle consists of egg (which is deposited inside a cocoon), juvenile, and reproductive hermaphrodite adult. Although most leeches typically secrete cocoons, there may be periods of the leech life cycle when it is a simultaneous hermaphrodite. Freshwater leeches most often breed in the spring, lay eggs in the summer, and overwinter on a host or in the benthos. The typical life span for leeches is one to three years.
The male reproductive system consists of four to nine pairs of testes (testisacs) and spermatozoa cemented together in seminal vesicles that enter two ejaculatory ducts that fuse to form an atrium. The male reproductive system is composed of vas deferens, epididymis, sperm-sacs, atrium, and a male gonopore. The female reproductive system consists of two ovaries (ovisacs) with oviducts that unite to form a uterus and a female gonopore. The morphology and arrangement of male and female reproductive systems are important taxonomic characters. In most leeches, spermatophores are released onto the body surface of the partner and enter tegument via histolytic enzymes; here, they migrate through the coelom to fertilize oocytes within the female ovaries. However, in leeches of the family Hirudinidae, the penis places sperm within the female gonopore. Like their supposed earthworm cousins, leeches use a clitellum (a region of thickened tegument) to hold their eggs and secrete a cocoon. The clitellum is inconspicuous and secretes rings that collect fertilized ova as they pass anteriorly over the female gonopore and are sloughed off the anterior end of the body as cocoons (usually from May to August). Mating involves the intertwining of bodies, during which each deposits sperm in the others’ clitellar area. The rhyncobdellid leeches have no penis but produce sharp packages of sperm which are forced through the body wall. Young leeches hatch and exit the cocoon, and development usually takes some years to complete.
Leeches have an open circulatory system, and the respiratory system is simple, with respiration taking place through the body wall; a slow undulating movement observed in some leeches is said to assist gaseous exchange. Aquatic leeches often move to the surface when in water of low oxygen content.
Leech sensory organs are on the head and body surface, allowing it to detect changes in light intensity, temperature, and vibration. Chemical receptors on the head provide a sense of smell, and there may be one or more pairs of ocelli (eye-spots) on the first few anterior segments. The sensory structures are typically arranged metamerically and include both sensillae, and touch/pressure sensors. These structures allow leeches to detect chemicals, heat, light, pressure, touch, and disturbances in water. The number of ocelli and their arrangement may be of some use in leech classification.
Leeches serve as vectors for numerous hematozoan (intraerythrocytic) parasites, including the protists Babesiosoma, Dactylosoma, and Haemogregarina. In Arkansas, hematozoans (Haemogregarina spp.) have been reported from several turtles of the families Chelydridae, Emydidae, and Kinosternidae, but, to date, no life cycle studies have been conducted on Arkansas hosts and their leeches.
Leeches (Piscicolidae) have also been reported on marine turtles. The Green sea turtle leech, Ozobranchus brachiatus, usually infects the green sea turtles (Chelonia mydas) but has also been reported from hawksbill sea turtle (Eretmochelys imbricata). Massive infection of Ozobranchus margoi on loggerhead sea turtles (Caretta caretta) has been reported to cause sea turtle leech erosion disease that presents with severe skin lesions, deep cutaneous erosion, eye injuries, and even host death. In addition, another potential hazard associated with Ozobranchus spp. infection is the risk of chelonid herpesvirus 5 transmission. This virus is associated with the development of fibropapillomatosis, a pathological condition of sea turtles characterized by occurrences of debilitating tumors in the skin and internal organs, which can progress to death.
The Order Acanthobdellida consists of leeches with twenty-nine segments and is a primitive taxon with one genus, Acanthobdella, on salmonid and thymallid (coldwater) fishes in Europe, particularly those from Lake Baikal in Russia. Acanthobdella peledina is widespread and found in boreal regions from Scandinavia, throughout Siberia and Alaska, and Acanthobdella livanowi is restricted to the Kamchatka Peninsula of extreme eastern Siberia. These leeches have no anterior sucker, but setae are present, and the coelom comprises compartments in five anterior segments.
The Order Arhynchobdellida includes about five families that are aquatic or terrestrial. They have a large mouth opening, non-eversible muscular ridged pharynx, five annuli per segment, with three pairs of jaws or without jaws (armed with teeth), and large testes arranged in metameric pairs. The family Erpobdellidae is the largest family of North American leeches, and three species occur in Arkansas, including Erpobella fervida, E. microstoma, and E. punctata. All are predatory or scavengers on oligochaetes, aquatic insect larvae, snails, and small crustaceans. Other genera in this order include Barbronia, Hirudo, Limnatis, Macrobdella, Motobdella, and Philobdella. Notable species include H. medicinalis, Hirundinaria granulosa (the Indian medical leech), H. zeylanica (the terrestrial leech that attacks humans and other animals in the tropics of Asia), and Limnatis nilotica (the large horse leech in Europe). Members of the genus are sometimes accidently ingested by various animals and attach and suck blood along the esophagus and nasopharyngeal area. The European medicinal leech has a tripartite jaw filled with hundreds of tiny, sharp teeth. The incision mark left on human skin is an inverted Y inside a circle. The North American counterpart is Macrobdella decora, a much less efficient medical leech. Others in this order include the family Hirudinidae, characterized by aquatic leeches, and the family Haemadipsidae by terrestrial leeches. In the latter are Haemadipsa sylvestris (the Indian leech) and H. zeylanica (the Japanese mountain or land leech).
The order Rhynchobdellida includes the “jawless leeches” that are all aquatic with a small mouth (without jaws or dentition as a pore), and an eversible (extendable) proboscis derived from the pharynx. They have a muscular, straw-like proboscis puncturing organ in a retractable sheath. Typical genera include Glossiphonia, Haementeria, and Placobdella. Haementeria officinalis is the medicinal leech of Central and South America, and Placobdella parasitica is the common North American turtle leech. There are two important families; the Glossiphoniidae are flattened leeches with poorly defined anterior suckers that live in freshwater habitats, and the family Piscicolidae are found in both marine and freshwater habitats and have cylindrical bodies and usually well-marked, bell-shaped, anterior suckers. Other important North American genera include Actinobdella, Alboglossiphonia, Batracobdella, Cystobranchus, Helobdella, Marvinmeyeria, Myzobdella, Piscicola, and Theromyzon.
Little study occurred on leeches in Arkansas until research exploded in the late twentieth and early twenty-first centuries. Twenty-two species from five families (Erpobdellidae, Glossiphoniidae, Haemopidae, Hirudinidae, Piscicolidae) have been reported from northern Arkansas, but no summaries of leeches from the southern part of the state are yet available. Fish leeches of the families Piscicolidae (Cystobranchus verrilli, Gonimosobdella klemmi, Myzobdella spp.) and Glossiphoniidae (Actinobdella inequiannulata, Placobdella montifera) have been reported in the state from various fishes. On amphibians of Arkansas, reports of new records of leeches on various salamanders and frogs, including Placobdella cryptobranchii on Ozark hellbenders (Cryptobranchus alleganiensis bishopi); Placobdella picta on spotted salamanders (Ambystoma maculatum), American toads (Anaxyrus americanus), southern leopard frogs (Lithobates sphenocephalus utricularius), and wood frogs (Lithobates sylvaticus); P. parasitica on central newts (Notophthalmus viridescens louisianensis), American bullfrogs (Lithobates catesbeianus), green frogs (Lithobates clamitans), common snapping turtles (Chelydra serpentina) and eastern river cooters (Pseudemys concinna); and Macrobdella diplotertia on spotted salamanders (Ambystoma maculatum) and wood frogs (Lithobates sylvaticus). Leeches are common on North American turtles, and in Arkansas, Placobdella multilineata has been reported on razor-backed musk turtles, Sternotherus carinatus. No leeches have been reported from lizards, snakes, alligators, birds, or non-human mammals from Arkansas. However, two species of leeches have been reported from the American alligator, Alligator mississippiensis in Mississippi, P. multilineata and the recently described P. siddalli.
Additional research is justified to help elucidate the leech fauna of the state, as many physiographic regions and potential hosts have not yet been completely surveyed. Additional species surely occur in Arkansas, including eight species reported from adjacent states, but they have not yet been collected.
For additional information:
Adam, Robert, and Peter Zakrzewski. “Therapeutic Use of Leeches: From the “Annelids” or Medicine.” University of Toronto Medical Journal 79 (2001): 65–70.
Briggler, Jeffrey T., Kenton M. Lohraff, and Ginny L. Adams. “Amphibian Parasitism by the Leech Desserobdella picta at a Small Pasture Pond in Northwest Arkansas.” Journal of Freshwater Ecology 16 (2001): 105–111.
Burreson, Eugene M., and D. W. Williams. “Redescription of Cystobranchus virginicus Hoffman, 1964, and Cystobranchus salmositicus (Meyer, 1946) (Hirudinida: Piscicolidae) from Freshwater Fishes in North America.” Comparative Parasitology 72 (2005): 157–165.
Curry, M. G. “Three Leeches (Hirudinea) New to Arkansas with Ecological and Distribution Notes.” Wasmann Journal of Biology 34 (1976): 5–8.
Davies, R. W., and C. G. Chapman. “First Record from North America of the Piscicolid Leech, Ozobranchus margoi, a Parasite of Marine Turtles.” Journal of the Fisheries Research Board of Canada 31 (1974):104–106.
Davies, R. W., F. R. Govedich, and William E. Moser. “Leech Parasites of Birds.” In Parasitic Diseases of Wild Birds, edited by C. T. Atkinsen, N. J. Thomas, and D. B. Hunter. Hoboken, NJ: Wiley-Blackwell, 2008.
Hyson, John M. “Leech Therapy: A History.” Journal of the History of Dentistry 53 (2005): 25–27.
Klemm, Donald J. “Freshwater Leeches.” In A Guide to the Freshwater Annelida (Polychaeta, Naidid and Tubificid Oligochaeta, and Hirudinea) of North America, edited by Donald J. Klemm. Dubuque: Kendall/Hunt Publishing Company, 1985.
———. “Hirudinea.” In Freshwater Macroinvertebrates of Northeastern North America, edited by B. L. Peckarsky, P. R. Fraissinet, M. A. Penton, and D. J. Conklin Jr. Ithaca, NY: Cornell University Press, 1990.
McAllister, Chris T., Charles R. Bursey, Henry W. Robison, and Michael A. Barger. “Haemogregarina sp. (Apicomplexa: Haemogregarinidae), Telorchis attenuata (Digenea: Telorchiidae) and Neoechinorhynchus emydis (Acanthocephala: Neoechinorhynchidae) from Map Turtles (Graptemys spp.), in Northcentral Arkansas.” Journal of the Arkansas Academy of Science 68 (2014): 154–157. Online at http://scholarworks.uark.edu/cgi/viewcontent.cgi?article=1240&context=jaas (accessed September 14, 2018).
McAllister, Chris T., Matthew B. Connior, Henry W. Robison, Thomas J. Fayton, Renn Tumlison, and Stanley E. Trauth. “Hematozoan Parasites (Apicomplexa: Kinetoplastida) of Seven Arkansas Reptiles (Testudines, Ophidia).” Journal of the Arkansas Academy of Science 70 (2016): 273–278. Online at http://scholarworks.uark.edu/cgi/viewcontent.cgi?article=2216&context=jaas (accessed September 14, 2018).
McAllister, Chris T., David Jamieson, and Mark E. Siddall. “Natural History Notes: Notophthalmus viridescens louisianensis (Central Newt).” Leech Infestation. Herpetological Review 39 (2008): 205–206.
McAllister, Chris T., and William E. Moser. “Natural History Notes: Sternotherus carinatus (Razor-backed Musk Turtle). Ectoparasites.” Herpetological Review 43 (2012): 128.
McAllister, Chris T., William E. Moser, and Donald J. Klemm. “Actinobdella inequiannulata (Annelida: Hirudinida: Rhynchobdellida: Glossiphonidae) from White Crappie, Pomoxis annularis (Perciformes: Centrarchidae), in Arkansas, U.S.A.” Comparative Parasitology 78 (2011): 392–394.
McAllister, Chris T., William E. Moser, and Dennis J. Richardson. “Placobdella papillifera (Annelida: Hirudinida: Glossiphoniiidae) Infesting the Stinkpot, Sternotherus odoratus (Testudines: Kinosternidae): New State Record for Oklahoma.” Proceedings of the Oklahoma Academy of Science 93 (2013): 29–31.
McAllister, Chris T., William E. Moser, Dennis J. Richardson, and Henry W. Robison. “New Host and Geographic Distribution Record for the Leech, Myzobdella reducta (Annelida: Hirudinida: Rhynchobdellida: Piscicolidae), from Arkansas.” Journal of the Arkansas Academy of Science 66 (2012): 190–192. Online at http://scholarworks.uark.edu/cgi/viewcontent.cgi?article=1326&context=jaas (accessed September 14, 2018).
McAllister, Chris T., Steve J. Upton, Stanley E. Trauth, and Charles R. Bursey. “Parasites of Wood Frogs, Rana sylvatica from Arkansas, with a Description of a New Species of Eimeria (Apicomplexa: Eimeriidae).” Journal of the Helminthological Society of Washington 62 (1995): 143–149.
McCallum, Malcolm L., William E. Moser, Benjamin A. Wheeler, and Stanley E. Trauth. “Amphibian Infestation and Host Size Preference by the Leech Placobdella picta (Verrill, 1872) (Hirudinida: Rhynchobdellida: Glossiphoniidae) from the Eastern Ozarks, USA.” Herpetology Notes 4 (2011): 147–151.
Moser, William E., Donald J. Klemm, Dennis J. Richardson, Benjamin A. Wheeler, Stanley A. Trauth, and Bruce A. Daniels. “Leeches (Annelida: Hirudinida) of Northern Arkansas.” Journal of the Arkansas Academy of Science 60 (2006): 84–95. Online at http://scholarworks.uark.edu/cgi/viewcontent.cgi?article=1502&context=jaas (accessed September 14, 2018).
Moser, William E., Dennis J. Richardson, Chris T. McAllister, Jeffrey T. Briggler, Charlotte I. Hammond, Stanley E. Trauth, and Henry W. Robison. “New Host and Distributional Records of the Leech Placobdella multilineata Moore, 1953 (Hirudinida: Glossophoniidae).” Journal of the Arkansas Academy of Science 68 (2014): 163–166. Online at http://scholarworks.uark.edu/cgi/viewcontent.cgi?article=1242&context=jaas (accessed September 14, 2018).
Moser, William E., Dennis J. Richardson, Benjamin A. Wheeler, Kelly J. Irwin, Bruce A. Daniels, Stanley E. Trauth, and Donald J. Klemm. “Placobdella cryptobranchii (Rhynchobdellida: Glossiphoniidae) on Cryptobranchus alleganiensis bishopi (Ozark Hellbender) in Arkansas and Missouri.” Comparative Parasitology 75 (2008): 98–101.
Richardson, Dennis J., Chris T. McAllister, Andrew J. Heaton, Eric E. Pulis, and William E. Moser. “Placobdella multilineata Moore, 1953 (Hirudinida: Glossophoniidae) from the Gulf Coast Box Turtle, Terrapene carolina major (Agassiz, 1857) (Testudines: Emydidae) in Mississippi, U.S.A.” Comparative Parasitology 83 (2016): 272–274.
Richardson, Dennis J., William E. Moser, Charlotte I. Hammond, Eric A. Lazo-Wasem, Chris T. McAllister, and Eric E. Pulis. “A New Species of Leech of the Genus Placobdella (Hirudinida, Glossiphoniidae) from the American Alligator (Alligator mississippiensis) in Mississippi, USA.” Zookeys 667 (2017): 39–49.
Richardson, Dennis J., William E. Moser, Chris T. McAllister, Renn Tumlison, Charlotte I. Hammond, Henry W. Robison, and David A. Neely. “New Host and Geographic Distribution Records for the Fish Leech Myzobdella reducta (Meyer, 1940) (Hirudinida: Piscicolidae).” Journal of the Arkansas Academy of Science 68 (2014): 167–169. Online at http://scholarworks.uark.edu/cgi/viewcontent.cgi?article=1243&context=jaas (accessed September 14, 2018).
Richardson, Dennis J., Renn Tumlison, J. W. Allen, Jr., William E. Moser, Chris T. McAllister, Stanley E. Trauth, and Henry W. Robison. “New Host Records for the Fish Leech Cystobranchus klemmi (Hirudinida: Piscicolidae) on Cyprinid Fishes from Arkansas and Oklahoma.” Journal of the Arkansas Academy of Science 67 (2013): 211–213. Online at http://scholarworks.uark.edu/cgi/viewcontent.cgi?article=1287&context=jaas (accessed September 14, 2018).
Siddall, Mark E., and Sherwin S. Desser. “Alternative Leech Vectors for Frog and Turtle Trypanosomes.” Journal of Parasitology 78 (1992): 562–563.
Thigpen, Christopher S., Stanley E. Trauth, L. I. Bagwell, John D. Konvalina, S. A. Schratz, Chris T. McAllister, William E. Moser, Dennis J. Richardson, and Henry W. Robison. “New Host and County Records of the Fish Leech Cystobranchus klemmi (Hirudinida: Piscicolidae) in Arkansas.” Journal of the Arkansas Academy of Science 69 (2015): 147–148. Online at http://scholarworks.uark.edu/cgi/viewcontent.cgi?article=1029&context=jaas (accessed September 14, 2018).
Trauth, Stanley E., and R. G. Neal. “Geographic Range Expansion and Feeding Response by the Leech Macrobdella diplotertia (Annelida: Hirudinea) to Wood Frog and Spotted Salamander Egg Masses.” Journal of the Arkansas Academy of Science 58 (2004):139–141. Online at http://scholarworks.uark.edu/cgi/viewcontent.cgi?article=1586&context=jaas (accessed September 14, 2018).
Turbeville, J. M., and Jeffrey T. Briggler. “The Occurrence of Macrobdella diplotertia (Annelida: Hirudinea) in the Ozark Highlands of Arkansas and Preliminary Observations on its Feeding Habits.” Journal of Freshwater Ecology 18 (2003): 155–159.
Williams, J. I., and Eugene M. Burreson. “Gonimosobdella klemmi n. gen. sp. (Hirudinida: Piscicolidae) from Cyprinid Fishes in Arkansas, Illinois, and Missouri, U.S.A.” Comparative Parasitology 72 (2005): 166–172.
Wirchansky, B. A., and D. H. Shain. “A New Species of Haemopis (Annelida: Hirudinea): Evolution of North American Terrestrial Leeches.” Molecular Phylogenetics and Evolution 54 (2010): 226–234.
Chris T. McAllister
Eastern Oklahoma State College
 Fish Leeches
Fish Leeches 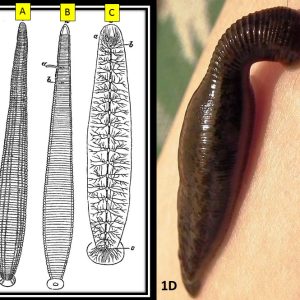 Medicinal Leech
Medicinal Leech  Turtle Leeches
Turtle Leeches  Turtle Leeches
Turtle Leeches 




Comments
No comments on this entry yet.