calsfoundation@cals.org
Lice
Lice belong to the Phylum Arthropoda, Class Insecta, Order Phthiraptera, with four suborders: Anoplura (sucking lice), occurring on mammals exclusively; Rhynchophthirina, parasites of elephants and warthogs; Ischnocera, which are mostly avian lice, though one family parasitizes mammals; and Amblycera, a primitive suborder of chewing lice, widespread on birds but also infesting South American and Australasian mammals. The chewing lice (suborders Rhynchophthirina, Ischnocera, and Amblycera) were previously combined in the order Mallophaga, which did not reflect natural grouping. There are nearly 5,000 described species of lice, with about 4,000 being parasitic on birds and 800 on mammals, within about twenty-six families of described species of phthriapterans. Many mammal species can be infested by sucking lice, including seals and walruses. These “marine lice” all belong to the family Echinophthiriidae, and they can live for long periods under water by taking a layer of air down with them between their specially modified body setae (“hairs”) or by breathing air trapped in the host’s body hair. Lice occupy every continent in all the habitats that their host animals are found, even in the Antarctic. In Arkansas, lice have been reported from endemic pocket gophers and other animals; they also pose problems for poultry farmers and infest pets and humans.
Evolutionarily, the order is a monophyletic grouping and has traditionally been divided into two suborders, the sucking lice (Anoplura) and the chewing lice (Mallophaga); however, recent classifications show that the Mallophaga are paraphyletic, and the four suborders above are now recognized. It is assumed that the order is derived from a primitive pscopteran-like ancestor that became parasitic first on birds. Human head and body lice (genus Pediculus) share a common ancestor with chimpanzee lice, while pubic lice (genus Phthirus) share a common ancestor with gorilla lice. The familial relationships among most major groups of lice are poorly understood, and resolution of these relationships awaits analyses of morphological and molecular data.
English entomologist George Henry Evans Hopkins (1898–1973) made major contributions in the study of lice. Three leading modern researchers are Ricardo Palma (National Museum of New Zealand), who mainly works on chewing lice; Lance A. Durden (Georgia Southern University), who is an expert of sucking lice; and Vincent S. Smith (Natural History Museum, London), who studies the coevolution of lice with their avian and mammalian hosts.
Generally, lice are wingless, dorsoventrally flattened ectoparasites with three nymphal instars. Compound eyes are reduced or (mostly) absent with no ocelli. They have simple or gradual metamorphosis with no pupal stage. Their mouthparts are modified for either chewing or as stylets in sucking lice. In anoplurans, some tibiae and tarsi are modified as tibio-tarsal claws for gripping individual host hairs. Reduced labial palps are present, as well as three to five segmented antennae. In the Amblycera, antennae are recessed into grooves long the side of the head, filiform in the Ischnocera (may be modified as clasping organs in males), and typically short in the Anoplura.
Interestingly, evidence of cophylogeny (the simultaneous evolutional development) between pocket gophers (Geomyidae) and chewing lice (Geomydoecus and Thomomydoecus spp.) has become so conclusive that the gopher-louse system is now perhaps the preeminent “textbook example” of cophylogeny. These genera are found exclusively on pocket gophers. The parasite’s entire life cycle takes place on its host, and lice cannot live for extended periods of time off their host. More than 120 species of chewing lice have been described from pocket gophers. In Arkansas, these lice have been reported from endemic pocket gophers (Geomys) from Izard County, commonly called the Ozark pocket gopher.
The Amblycera primarily ingest feathers or hair, and some live off epithelial cells, mucus, and sebaceous secretions. In addition, a few chew into the quills of birds, feed on blood, and may cause dermal lesions, feather or hair damage, irritation, and restlessness. These lice are wingless, dorsoventrally flattened with no ocelli, and they possess short, club-shaped antennae (with three to five segments). The head is usually broader than the prothorax, mandibles (and maxillary palps) are conspicuous, and the maxillae and labium are reduced. These lice tend to be generalists, and many species are not host specific.
The suborder includes about six families, including the Boopidae (about seven genera, mostly on Australian marsupials and cassowarys), Gyropidae (about nine genera on Central and South American rodents and marsupials), Laemobothriidae (a single genus on birds), Menoponidae (about seventy-two genera, all on birds), Ricinidae (about three genera, all on birds), and Trimenoponidae (about five genera, on Central and South American rodents and marsupials). Some of the representative species are Ricinus bombycillae (from Bohemian waxwing), Trinoton anserinum (from mute swan), Gliricola porcelli and Gyropus ovalis (on guinea pigs), Menacanthus stramineus (the body louse of chickens and turkeys, which is about three millimeters long and may occur with up to 35,000 per bird, prefers skin, and can cause irritation severe enough to disrupt the bird’s feeding), and Menopon gallinae (the shaft louse of galliform birds, especially domestic chickens). When any of these lice become a problem for an Arkansas poultry producer, the University of Arkansas Cooperative Extension Service recommends using Rabon 50% WP (tetrachlorvinphos) and Ravap EC (23% tetrachlorvinphos and 5.3% dichlorvos) on walls, roosts, cracks, crevices, and interiors thoroughly, and spraying birds lightly. There is also no withholding period from last application to slaughter.
The Ischnocera are permanent obligatory ectoparasites of birds and mammals, and the group has a cosmopolitan distribution. It includes over 3,000 species and is composed of two families, the Trichodectidae (approximately twenty genera, all on mammals) and the Philopteridae (about 134 genera, all on birds). Some authors suggest dividing these two families into five different families. These lice do not have maxillary palps, but their slender filiform antennae are conspicuous and stick out prominently from the side of head with three or five segments. The food items of this suborder are confined to feathers or hair, and some species on birds sometimes confine themselves to specific sites on the host body. They are also species-specific on hosts. Some representative species are Anaticola anseris and A. crassicornis (duck lice), Bovicola bovis (cattle biting louse), B. equi (horse biting louse), Columbicola columbae (slender pigeon louse), Cuclotogaster heterographus (chicken head louse), Felicola subrostratus (cat louse), Goniocotes bidentatus (pigeon louse), G. gallinae (fluff louse of chickens), G. gigas (giant chicken louse), Goniodes dissimilis (reddish-brown chicken louse), Lipeurus caponis (wing louse), Oxylipeurus polytrapzius (slender turkey louse), Strigiphilus garylarsoni (owl louse), Trichodectes canis (dog biting louse), and T. tibalis (on deer in the western United States). Irritation caused by T. canis can be severe, especially on puppies, and T. canis is also an important intermediate host of the double-pored dog tapeworm, Dipylidium caninum. Another species, Bovicola limbata, is an ischnoceran louse of goats. It is sexually dimorphic, with the male being smaller than the female.
The Rhynchophthirina comprises only three known African/Asian species in the genus Haematomyzus (Family Haematomyzidae). Maxillary palps are absent, and the chewing mandibles are at the end of projecting structure; all feed on blood. The three species are Haematomyzus elephantis from both African (type host) and Asian elephants, H. hopkinsi from the warthog, and H. porci from the bush pig.
Members of the Anoplura (sucking lice) infest mammals only and ingest blood. There are more than 550 described species, with seventy-seven species known from North America. They are 0.45–8.0 mm long as adults, wingless, and dorsoventrally flattened; their head is narrower than the prothorax, and eyes are absent or present (no ocelli). The antennae are conspicuous and composed of three to five segments (scape, pedicel, with a flagellum divided into three to five sub-segments). Sucking lice are small wingless insects, and their mouthparts, which are retractable into their head, are adapted for piercing and sucking. There is a cibarial pump at the start of the gut; it is powered by muscles attached to the inside of the cuticle of the head. The mouthparts consist of a proboscis that is toothed and a set of stylets arranged in a cylinder inside the proboscis, containing a salivary canal and a food canal. Some representative species are Haematopinus asini (horse sucking louse), H. eurysternus (short-nosed cattle louse), H. quadripertusus (cattle tail louse), H. suis (hog louse), H. ventricosus (rabbit louse), Hoplopleura pacificus (on peridomestic rats in the warmer parts of the world including the southern United States), L. setosus (dog sucking louse), Solenopotes capillatus (little blue cattle louse), Pediculus humanus humanus (human body louse), P. humanus capitus (human head louse), Pediculus mjobergi (found on New World monkeys), Polyplax spinulosa (spined rat louse, which may transmit epidemic typhus bacteria between rats), Phthirus gorillae (on gorillas), and P. pubis (pubic louse of humans). In the family Polyplacidae, Neohaematopinus sciuropteri is a vector of the zoonotic agent of sporadic epidemic typhus (caused by certain strains of Rickettsia prowazekii) to flying squirrels (Glaucomys spp.), which act as reservoir hosts for this agent.
Reproductively, sucking lice eggs (nits) are usually cemented to the hair of the host. A typical life cycle includes eggs attached to the hair although the body louse of humans cements its eggs to clothing fibers. The nymph hatches three to fourteen days later, and there are three nymphal instars (about one week each), followed by molting into an adult, then mating. Lice inhabiting birds, however, may simply leave their eggs in parts of the body inaccessible to preening, such as the interior of feather shafts.
Pediculosis (louse infestation) dates back to prehistory. The oldest known fossils of louse eggs, approximately 10,000 years old, were found in Brazil. Lice have also been discovered from hair combs in Israel dating back 2,000 years. There are many historic human infestations of head and body lice. They were ubiquitous in human society until at least the Middle Ages (from the fifth to the fifteenth century). Robert Hooke’s 1667 book Micrographia showed a louse grasping a human hair. The head louse of humans (Pediculus humanus capitis) was drawn in the seventeenth century by Hooke. Louse-borne infectious diseases affected nearly one-third of Napoleon’s soldiers, meaning that these diseases might have been a major factor in the French retreat from Russia. Soldiers in the trenches of the World War I suffered severely from lice and the epidemic typhus bacteria they carried. The Germans bragged that they had lice under effective control but themselves suffered tremendously from them in World War II, particularly on the Eastern Front in Russia.
In forensic entomology, lice can be used as indicators of contact with another person. Human lice can be easily transferred between individuals, and identification of DNA of multiple individuals using blood meals from body and head lice has been demonstrated in laboratory settings.
Lice can harm animal populations and, if the infestation is heavy enough, may even reduce host life expectancy. However, most infestations appear to have little effect on their host. The habit of dust bathing in domestic chicken hens is probably an attempt by the birds to rid themselves of lice. In addition, ischnocerans may reduce the thermoregulation effect of the plumage; thus, heavily infested birds lose more heat than others. Human body lice (P. h. humanus) can be the vector of Rickettsia prowazekii, which causes epidemic (louse-borne) typhus; the bacterium Bartonella quintana, which causes trench fever, a nonfatal, debilitating disease; and Borrelia recurrentis, which causes relapsing fever, with a mortality rate of up to fifty percent in some populations. Crab lice, P. pubis (considered a sexually transmitted disease), inhabit hair of the genitalia and also the armpits, beard, mustache, eyebrows, eyelashes, and eyelids (Phthiriasis palpebrarum). Their life cycle is less than one month, and a single female can lay up to ninety nits during her lifetime. Today, the prevalence of human louse infestation, especially of head lice, is still very high worldwide.
Human lice infestations can be controlled with louse combs and prescription lotions (ivermectin, malathion) or over-the-counter medications with pyrethrins, permethrins, or gamma isomers of hexachlorobenzene or medicated anti-lice shampoos (Liceadex or Nix). However, strains of head lice resistant to over-the-counter treatments were found in 2015 in twenty-five U.S. states, including Arkansas. Arkansans were told to turn to prescription treatments instead of using treatments off the shelf. In addition, one study revealed that ninety-eight percent of head lice have developed a resistance to pyrethrins and permethrins, the active ingredients in most remedies available in pharmacies. Lice of pets can be controlled by dips and insecticidal dusts and ear tags for domestic animals impregnated with permethrin or cypermethrin. There are no commercial vaccines against louse-borne diseases of humans. Single-dose oral administration of the antibiotic doxycycline is most effective in treating or preventing epidemic typhus when permethrin dusting of clothing for louse control is not possible.
Little has been published on the lice of Arkansas. Two species of sucking lice, Neohematopinus sciurinus and Neohaematopinus sciuropteri, have been reported from sciurid rodents from Arkansas. In addition, two new species of lice (Myrsidea bensoni and Brueelia limnothlypiae) were described from Swainson’s warblers (Limnothlypis swainsonii in the state.
The International Society of Phthirapterists includes about 100 clinicians, evolutionary biologists, veterinary scientists, and taxonomists working on various aspects of parasitic louse biology. The society aims to advance the study of Phthiraptera and louse-borne diseases in all its aspects of theory, principles, methodology, and practice.
For additional information:
Demastes, James W., and Mark S. Hafner. “Cospeciation of Pocket Gophers (Geomys) and Their Chewing Lice (Geomydoecus).” Journal of Mammalogy 74 (1993): 521–530.
Durden, Lance A. “A New Species and an Annotated World List of the Sucking Louse Genus Neohaematopinus (Anoplura: Polyplacidae).” Journal of Medical Entomology 28 (1991): 694–700.
Durden, Lance A., and John E. Lloyd. “Lice (Phthiraptera).” In Medical and Veterinary Entomology, 2nd ed., edited by G. R. Mullen and L. A. Durden. Amsterdam: Elsevier, 2009.
Durden, Lance A., and Guy G. Musser. “The Sucking Lice (Insecta, Anoplura) of the World: A Taxonomic Checklist with Records of Mammalian Hosts and Geographical Distributions.” Bulletin of the American Museum of Natural History 218 (1994): 1–90.
Elrod, Douglas A., Gary A. Heidt, Diana M. A. Elrod, Melaney Birdsong, and Earl G. Zimmerman. “A Second Species of Pocket Gopher in Arkansas.” Southwestern Naturalist 41 (1996): 395–398.
Hafner, Mark S., and R. D. M. Page. “Molecular Phylogenies and Host-Parasite Cospeciation: Gophers and Lice as a Model System.” Philosophical Transactions of the Royal Society of London B 349 (1995):77–83.
Hopkins, George Henry Evans. “The Distribution of Phthiraptera on Mammals.” In First Symposium on Host Specificity Amongst Parasites of Vertebrates, edited by J. G. Baer. Neuchatel, Switzerland: Institute of Zoology, University of Neuchatel, 1957.
“Insecticide Recommendations for Arkansas.” University of Arkansas Cooperative Extension Service. MP 144 (2017). Online at: https://www.uaex.edu/publications/pdf/mp144/MP144.pdf (accessed January 29, 2018).
Kim, Ke Chung, H. D. Pratt, and C. J. Stanojanovich. The Sucking Lice of North America: An Illustrated Manual for Identification. University Park: Pennsylvania State University Press, 1986.
Light, Jessica E., J. M. Allen, L. M. Long, T. E. Carter, L. Barrow, G. Suren, D. Raoult, and D. L. Reed. “Geographic Distribution and Origins of Human Head Lice (Pediculus humanus capitis) Based on Mitochondrial Data”. Journal of Parasitology 94 (2008): 1275–1281.
McAllister, Chris T., Matthew B. Connior, and Lance A. Durden. “Ectoparasites of Sciurid Rodents in Arkansas, Including New State Records for Neohaematopinus spp. (Phthiraptera: Anoplura: Polyplacidae).” Journal of the Arkansas Academy of Science 67 (2013): 197–199. Online at: http://scholarworks.uark.edu/jaas/vol67/iss1/34/ (accessed January 29, 2018).
Mumcuoglu, Kosta Y. “Prevention and Treatment of Head Lice in Children.” Pediatric Drugs 1 (1999): 211–218.
Nadler, Steven A., Mark S. Hafner, John C. Hafner, and David J. Hafner. “Genetic Differentiation among Chewing Louse Populations (Mallophaga: Trichodectidae) in a Pocket Gopher Contact Zone (Rodentia: Geomyidae).” Evolution 44 (1990): 942–951.
Nuttall, G. H. F. “The Biology of Phthirus pubis.” Parasitology 10 (1918): 383–405.
Price, R. D., R. A. Hellenthal, and R. L. Palma. “World Checklist of Chewing Lice with Host Associations and Keys to Families and Genera.” In The Chewing Lice: World Checklist and Biological Overview, edited by R. D. Price. Illinois Natural History Survey Special Publication 24 (2003).
Roberts, Larry S., and John Janovy Jr. Foundations of Parasitology. 9th ed. Boston: McGraw-Hill Higher Education, 2012.
Rózsa, Lajos. “Patterns in the Abundance of Avian Lice (Phthiraptera: Amblycera, Ischnocera).” Journal of Avian Biology 28 (1997): 249–254.
Smith, Vincent S. “Avian Louse Phylogeny (Phthiraptera: Ischnocera): A Cladistic Study Based on Morphology.” Zoological Journal of the Linnean Society 132 (2001): 81–144.
Valim, Michel P., and Bryan M. Reiley. “The Chewing Lice (Insecta, Phthiraptera) Fauna of the Swainson’s Warbler, Limnothlypis swainsonii (Aves, Parulidae).” Journal of Medical Entomology 52 (2015): 850–857.
Veracx, A., and D. Raoult. “Biology and Genetics of Human Head and Body Lice.” Trends in Parasitology 28 (2012): 563–571.
Chris T. McAllister
Eastern Oklahoma State College
 Science and Technology
Science and Technology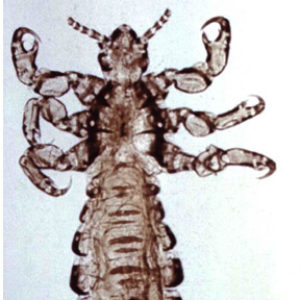 Lice
Lice 




Comments
No comments on this entry yet.