calsfoundation@cals.org
Mites
Mites are small arthropods (Phylum Arthropoda) belonging to the Class Arachnida (Subclass Acari) with two or three Superorders as follows: Acariformes (or Actinotrichida), Parasitiformes (or Anactinotrichida), and Opilioacariformes. There are four Orders, Mesostigmata, Prostigmata, Orbatida, and Astigmata. Among the more well-known mites are ticks (Ixodida). There are an estimated 48,200 species of described mites. The phylogeny of the Acari is still debatable, and several different taxonomic schemes have been proposed for their classification. The diversity of the Acari is extraordinary, and its fossil history goes back to at least the early Devonian Period (about 419 million years ago). Mites are very common in Arkansas, with chiggers being a particular pest of humans during warmer months.
Historically, references to mites/chiggers go as far back as sixth-century China, and by 1733, the first recognition of trombiculid mites in North America was made. In 1758, Carolus Linnaeus (1707–1778) described Acarus batatas (now Eutrombicula batatas). However, most information about chiggers was discovered because they were infesting humans and serving as vectors or agents of disease (transmitting scrub typhus or chigger-borne rickettsiosis) that arose during and after World War II. Some famous acarologists of the past include Anthonie C. Oudemans (1858–1943), Louis W. Sambon (1865–1931), Henry Ellsworth Ewing (1883–1951), James M. Brennan (1905–1985), Donald M. Allred (1923–1996), Richard B. Loomis (1925–1985), and William J. Wrenn (1935–2012), all of whom published extensively on mites and chiggers. But the foremost researcher was Alex Fain (1912–2009), who authored about 1,160 peer-reviewed papers on the subject and described over 2,500 taxa of organisms. He also developed a massive collection of mites made up of around 100,000 microscopic slides and estimated to contain more than 30,000 specimens.
Most acarines are small (0.1–1.0 millimeters), but the largest Acari (red velvet mites) may reach lengths of 10–20 mm. Mites are among the most diverse and successful of all the invertebrates and have exploited a vast array of niches. Many species live in soil as decomposers; some are predatory, while others are parasitic, although the majority are harmless to humans. They live freely in the soil or water, but a large number of species also live as ectoparasites on plants, animals, and molds.
Economically, mites can have both positive and negative effects. Some parasitic species can cause serious losses in food production, while predatory mites have been successfully used to target pests in biological control programs; herbivorous mites have also been used to attack weeds. Damage to crops is perhaps the most costly economic effect of mites, especially by spider mites and their relatives, earth mites, thread-footed mites, and gall and rust mites. Some parasitic forms affect humans and other mammals, causing damage by their feeding or causing and contributing to allergies. They can even be vectors of diseases, such as scabies (caused by Sarcoptes scabiei), scrub or bush typhus, or Japanese river disease (transmitted by some species of trombiculid mites or chiggers, particularly Leptotrombidium deliense), which is considered a dangerous pest in East Asia and the South Pacific because it often carries Orientia tsutsugamushi and rickettsialpox (from the mite Liponyssoides sanguineus). The mites are infected by the Rickettsia passed down from parent to offspring before eggs are laid in a process called transovarial transmission.
Wounds from chigger bites include a complex combination of enzymatic effects, resulting in mechanical damage and a combination of allergic and immune responses, plus possible secondary bacterial infection; therefore, no one remedy works equally well for most people. Most commonly, the chiggers’ salivary digestive enzymes cause itchy welts. According to Mayo Clinic, the chiggers “fall off after a few days, leaving behind red, itchy welts,” which normally heal on their own within one to two weeks.
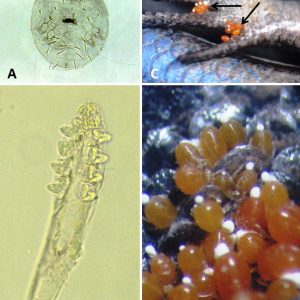
Most, if not all, mites are considered ectoparasites, meaning that they live on the outside of their hosts. A recent report found hundreds of Neottialges evansi (Hypoderatidae) mites behind the eyes of a double-crested cormorant, Phalacrocorax auritus. Deutonymphs of N. evansi are endoparasites (living inside their hosts), typically infecting fat deposits over the pectoral muscles, axillary areas, and vent of cormorants.
Most adult mites have four pairs of legs, but some have fewer. For example, gall mites (Phyllocoptes variabilis) have a worm-like body with only two pairs of legs; some parasitic mites have one pair or three pairs of legs in the adult stage. Pre-larval and larval stages have a maximum of three pairs of legs. The mouth parts of mites may be adapted for biting, stinging, sawing, or sucking. They breathe through tracheae, stigmata (small openings of the skin), intestines, and the skin itself. Species that hunt for other mites have very acute senses, but many mites are eyeless.
Mites of the Order Mesostigmata include about 100 families, about 900 genera, and over 8,000 species of both free-living and parasitic members (mainly on vertebrates). They possess heavily scleratinized bodies and a ventrally hidden hypostome without teeth. Haller’s organ (a sensory pit) is absent, but there is a pair of spiracles found between the second and fourth coxae. A tube (the tracheal trunk) usually extends anteriorly from each spiracle and can be seen through the cuticle; this is termed the peritreme. A bristle-like organ, the tritosternum, is usually present immediately ventral and behind the gnathostoma. One very important species in this order is the honeybee mite (Varroa destructor), a virulent parasite that infests its natural host, Apis cerana (Asian honeybee) in Asia and also Apis mellifera (western honeybees) worldwide. They feed off the bodily fluids of larval, pupal, and adult honeybees, and may carry viruses that are destructive to the bees, causing wing deformities; accordingly, these mites have been implicated in colony collapse disorder. Interestingly, some strains of honeybees have become resistant to Varroa, have developed Varroa-sensitive hygiene behavior, and can detect and remove Varroa in the brood. Another such parasitic mite is Acarapis woodi (family Tarsonemidae), which lives in the tracheae of honeybees. Hundreds of species are associated with other bees; however, most are poorly described. Interestingly, they attach to bees in a variety of ways. For example, worker bees of Trigona corvina, a species of stingless bee that lives primarily in Central and South America, have been found with mites attached to the outer face of their hind tibiae. Some that attach to bees are thought to be parasites, while others are beneficial symbionts.
Mites also parasitize some ant species, such as Eciton burchellii, a species of New World army ant. Others include the chicken mite (Dermanyssus gallinae), a cosmopolitan species commonly found on chickens and pigeons; the tropical rat mite (Ornithonyssus bacoti), a cosmopolitan species often found infesting mice in laboratory rodent colonies; and the canary lung mite (Sternostoma tracheacolum), another cosmopolitan species found in the respiratory tract of canaries.
The Order Prostigmata includes both poorly armored free-living and parasitic mites and chiggers possessing a ventrally hidden hypostome without teeth; Haller’s organ is absent, there is a pair of spiracles sometimes present, and, if so, they are either located between the chelicerae or on dorsal surface near center of body. Demodex mites (family Demodicidae) are parasites that live in or near the hair follicles of mammals, including humans. Some typical species include the dog follicular mite (Demodex canis), which may cause red mange in canids when secondary to Staphylococcus pyogenes–invaded lesions; there are many other cosmopolitan species of the genus in various animals (i.e., D. injai on dogs, D. folliculorum and D. brevis in humans, D. cati and D. gatoi on cats, D. equi on horses, and D. phylloides on pigs). Demodex mites have also been implicated in the human skin disease rosacea. Among the species that infest animals are members of the sarcoptic mange mites (family Sarcoptidae), which burrow under the skin.
Others include the sheep itch mite (Psorergates ovis), which can cause dermal problems in sheep; the straw itch mite (Pyemotes tritici) on grain beetles, which also may attack mammals; the common North American chigger, Eutrombicula cinnabaris (formerly E. alfreddugesi), which has brightly colored (red to orange) larval stages that feed on blood of reptiles (mostly lizards) with nymphs and adults being free-living, along with some species may transmit scrub typhus (a rickettsia); and Hannemania penetrans and H. dunni (trombiculid mites) with larval stages encysting in red or orange “mite pockets” in skin of salamanders, frogs, and toads in North America. In humans, chigger bites are most common on the lower parts of the body in areas of tight-fitting clothing, such as under socks.
Mites of the Order Orbatida (Cryptostigmata) include about 200 families, 1,200 genera, and 6,600 species. These mites are important decomposers, eating a wide variety of material including living and dead plant and fungal material, lichens, and carrion; some are predatory, although no oribatid mites are considered parasitic. Their feeding habits may differ between immatures and adults of the same species. Mites in this order are also called moss or beetle mites, and all free-living species possess a ventrally hidden hypostome without teeth; Haller’s organ and spiracles are absent. Stigmata and tracheae are usually present, and they open into a porous area. Mouthparts are drawn into a tube, the camerostome, which may have a hood-like sclerite covering it. Oribatid mites generally have a lower metabolism, a slow development, and a low fecundity. Species are iteroparous (multiple reproductive cycles over the course of its lifetime), with adults living a relatively long time. For example, in mites occurring in temperate forest soils, estimates of developmental time from egg to adult varies from several months to two years. These mites have six active instars: prelarva, larva, three nymphal instars, and the adult. All of these stages after the prelarva feed on a wide variety of material. The Oribatida are also of economic importance as intermediate hosts of various cestode (primarily anophlocephalid larval tapeworm) species, and by increasing the breakdown of organic material in the soil.
The Order Astigmata (itch or feather mites) includes both free-living and parasitic members. They are poorly sclerotized and possess a ventrally hidden hypostome without teeth, Haller’s organ, and spiracles; claws are absent, but they do have sucker-like structures on their pretarsi. These mites have no tracheal systems, and respiration is via tegumental respiration. Feather mites are found on almost all species of birds except penguins. Depending on the taxon to which they belong, they may variously feed on bacteria, feathers, fungus, skin flakes, and uropygial oil. The lifestyle of feather mites is affected by the microclimate (ambient temperature and relative humidity), like seasonal changes in temperature that can cause feather mites to shift their microhabitats on Eurasian blue tits (Cyanistes caeruleus); however, there is no evidence on microclimate affects diversity of mites. Specific examples include Chorioptes bovis, which causes mange in a variety of mammals; Knemidokoptes laevis, a depluming mite of chickens, pheasants, and ducks; K. mutans, the scaly-leg mite of chickens; Notoedres cati, the ear and facial mange in rodents, cats, and dogs; Psoroptes communis, which causes mange in a variety of mammals; and Otodectes cynotis, a mange mite of felids, canids, and mustelids that pierces skin but is non-invasive. One mite that causes problems for humans is the scabies or itch mite (S. scabiei) that invades and tunnels through the stratum corneum of the epidermis, causing intense dermatitis and skin rashes when the impregnated female tunnels into the skin and deposits eggs in the burrow. The larvae, which hatch in three to ten days, move about on the skin, molt into a nymphal stage, and then mature into adult mites. The adult mites live three to four weeks in the host’s skin. Other mammals, such as feral and domesticated dogs and cats (in which it is one cause of mange) as well as bovids, koalas, ungulates, wild boars, wombats, and great apes, are harmed by S. scabiei. Others that affect humans are the house mites Dermatophagoides pteronyssinus (European house-dust mite) and D. farinae (American house-dust mite). Both have worldwide distributions; live in beds, clothes, mattresses, carpets, and house dust; may cause several forms of allergic diseases, including hay fever, asthma, and eczema; and are known to aggravate atopic dermatitis. They feed mostly on dead skin and hair shed from humans.
Mites considered to be pests of plants include the gall mites (family Eriophyidae), the thread-footed mites (family Tarsonemidae), and the so-called spider mites (family Tetranychidae).
Mites are also important in forensic acarology or the utility and contribution of mites in criminal investigations, in illegal trade, and in cases of human and animal neglect. Several species feed on corpses, with mites of the genus Macrocheles common in the early stages of decomposition, while mites of the families Tyroglyphidae and Oribatidae (such as Rostrozetes) feed on dry skin in the later stages of decomposition.
There are several records of mites and chiggers from Arkansas vertebrates. The most common chigger of Arkansas reptiles is E. cinnabaris. It has been previously reported in the state from prairie lizards (Sceloporus consobrinus), eastern collared lizards (Crotaphytus collaris), five-lined skinks (Plestiodon fasciatus), broadhead skinks (Plestiodon laticeps), and six-lined racerunners (Aspidoscelis sexlineata). Additionally, the bird-voiced treefrog (Hyla avivoca) has been reported to be a rare host for E. cinnabaris—the first chigger reported to infest this frog. The most common chigger on Arkansas amphibians is H. dunni. They burrow intradermally in the stratum spongosium of the host. This chigger has been reported from Ouachita dusky salamanders (Desmognathus brimleyorum), Oklahoma salamanders (Eurycea tynerensis), Ozark zig-zag salamanders (Plethodon angusticlavius), Caddo Mountain salamanders (Plethodon caddoensis), Fourche Mountain salamanders (Plethodon fourchensis), Kiamichi Mountain salamander (Plethodon kiamichi), Rich Mountain salamanders (Plethodon ouachitae), pickerel frogs (Lithobates palustris), southern leopard frogs (Lithobates sphenocephalus utricularius), and dwarf American toads (Anaxyrus americanus charlesmithi). Dusky salamanders can have high infestation rates, which can lead to deformed limbs. Several chiggers/mites have been reported from Arkansas shrews, moles, and rodents, including Androlaelaps fahrenholzi, Echinonyssus blarinae, Euschoengastia peromysci, Glycyphagus hypudaei, Haemogamasus harperi, Laelaps kochi, Leptotrombidium peromysci, Listrophorus pitymys, Olistrophorus blarina, Ornithonyssus bacoti, and Protomyobia blarinae.
Unfortunately, little is known about the mites of birds in the state. However, one family (Pyroglyphidae or nest mites) occurs primarily in the nests of birds and other animals. These mites are mostly parasitic and consume blood, keratin, and skin. “Tropical fowl mite” is the common name used to describe the mite Ornithonyssus bursa from the family of mites Macronyssidae. This species of bird mite is widely distributed throughout warmer regions of the world. They are hematophagous, feeding on the blood of common birds, including pigeons, starlings, sparrows, Indian mynahs, poultry, and some wild birds. These mites are small with eight legs, barely visible to the eye, oval in shape with a sparse covering of short hairs, and extremely mobile. They are semitransparent in color, which makes them difficult to detect on skin until blood is ingested and then digested, when they may appear reddish to blackish.
Additional parasitic mites/chiggers have been reported from various Arkansas vertebrate hosts, including Acomatacarus polychaetus, A. whartoni, Androlaelaps casalis, Cheladonta ouachitensis, Hannemania multifemorala, Euschoengastia pipistrelli, E. setosa, Eutrombicula lipovskyana, Leptotrombidium myotis, Microtrombicula trisetica, Neoschongastia americana, Neotrombicula lipovskyi, N. richmondi, N. whartoni, and Parasecia gurneyi. However, much work remains to be accomplished by surveying potential hosts and more completely investigating the mite and chigger fauna of the state.
For additional information:
Andrews, R. M., J. McCarthy, J. R. Carapetis, and B. J. Currie. “Skin Disorders, Including Pyoderma, Scabies, and Tinea Infections.” Pediatric Clinics of North America 56 (2009): 1421–1440.
Anthony, Carl D., Joseph R. Mendelson III, and R. R. Simons. “Differential Parasitism by Sex on Plethodontid Salamanders and Histological Evidence for Structural Damage to the Nasolabial Groove.” American Midland Naturalist 132 (1994): 302–307.
Brennan, James M., and M. Lee Goff. “Keys to the Genera of Chiggers of the Western Hemisphere.” Journal of Parasitology 63 (1977): 554–566.
Connior, Matthew B., Lance A. Durden, and Chris T. McAllister. “Natural History Notes: Anaxyrus fowleri. Ectoparasites.” Herpetological Review 47 (2016): 104.
———. “New Records of Ectoparasites and Other Epifauna from Scalopus aquaticus and Blarina carolinensis in Arkansas.” Journal of the Arkansas Academy of Science 68 (2014): 137–139. Online at http://scholarworks.uark.edu/jaas/vol68/iss1/23/ (accessed February 25, 2021).
Connior, Matthew B., Lance A. Durden, Chris T. McAllister, R. Scott Seville, Charles R. Bursey, and Henry W. Robison. “New Records of Parasites (Apicomplexa, Acari, Anoplura, Nematoda) from Rodents in Arkansas.” Journal of the Arkansas Academy of Science 71 (2017).
Connior, Matthew B., Chris T. McAllister, Lance A. Durden, Stanley E. Trauth, and Henry W. Robison. “New Records of Chiggers (Acari) from Arkansas Amphibians (Caudata, Anura) and Reptiles (Sauria).” Journal of the Arkansas Academy of Science 70 (2016): 263–267. Online at http://scholarworks.uark.edu/jaas/vol70/iss1/43/ (accessed February 25, 2021).
Crossley, D. A., Jr. “Comparative External Morphology and Taxonomy of Nymphs of the Trombiculidae (Acarina).” University of Kansas Science Bulletin 40 (1960): 135–321.
Diaz, J. H. “Endemic Mite-Transmitted Dermatoses and Infectious Diseases in the South.” Journal of the Louisiana State Medical Society 162 (2010): 140–145, 147–149.
Loomis, Richard B. 1956. “The Chigger Mites of Kansas (Acarina, Trombiculidae).” University of Kansas Science Bulletin 37 (1956): 1195–1443.
McAllister, Chris T. “Observations on the Incidence of Chiggers, Eutrombicula alfreddugesi (Oudemans) on Crotaphytus (Sauria: Iguanidae) in Izard County, Arkansas.” Proceedings of the Arkansas Academy of Science 34 (1980): 125. Online at http://scholarworks.uark.edu/jaas/vol34/iss1/44/ (accessed February 25, 2021).
McAllister, Chris T., Charles R. Bursey, Henry W. Robison, and Matthew B. Connior. “Parasites of the Ozark Zig-Zag Salamander, Plethodon angusticlavius (Caudata: Plethodontidae), from Northern Arkansas.” Comparative Parasitology 80 (2013): 69–79.
McAllister, Chris T., Charles R. Bursey, Steve J. Upton, Stanley E. Trauth, and David B. Conn. “Parasites of Desmognathus brimleyorum (Caudata: Plethodontidae) from the Ouachita Mountains of Arkansas and Oklahoma.” Journal of the Helminthological Society of Washington 62 (1995): 150–156.
McAllister, Chris T., Stanley E. Trauth, and Charles R. Bursey. “Metazoan Parasites of the Graybelly Salamander, Eurycea multiplicata griseogaster (Caudata: Plethodontidae), from Arkansas.” Journal of the Helminthological Society of Washington 62 (1995): 66–69.
McAllister, Chris T., S. E. Trauth, and Charles R. Bursey. “Parasites of the Pickerel Frog, Rana palustris (Anura: Ranidae), from the Southern Part of its Range.” Southwestern Naturalist 40 (1995): 111–116.
McAllister Chris T., Steve J. Upton, and Stanley E. Trauth. “Endoparasites of Western Slimy Salamanders, Plethodon albagula (Caudata: Plethodontidae), from Arkansas.” Journal of the Helminthological Society of Washington 60 (1993): 123–126.
Perotti, M. A., and H. R. Braig. “Phoretic Mites Associated with Animal and Human Decomposition.” Experimental & Applied Acarology 49 (2009): 85–124.
Richardson, Dennis J., Lance A. Durden, and D. E. Snyder. “Ectoparasites of the Raccoon (Procyon lotor) from North-Central Arkansas.” Journal of the Kansas Entomological Society 67 (1994): 202–212.
Sambon, Louis W. “The Parasitic Acarians of Animals and the Part They Play in the Causation of the Eruptive Fevers and Other Diseases of Man. Preliminary Considerations Based upon an Ecological study of Typhus Fever.” Annals of Tropical Medicine and Parasitology 22 (1928): 67–132.
Sheehan, K. L., Greg S. Spicer, Barry M. O’Connor, and Ryan F. Hechinger. “No One Saw This Coming: Endoparasitic Mites Behind the Eyes of a Double-Crested Cormorant.” Journal of Parasitology 103 (2017): 295–297.
Tumlison, Renn, Matthew B. Connior, Henry W. Robison, Chris T. McAllister, Lance A. Durden, D. Blake Sasse, and David A. Saugey. “Vertebrate Natural History Notes from Arkansas, 2015.” Journal of the Arkansas Academy of Science 69 (2015): 106–115. Online at http://scholarworks.uark.edu/jaas/vol69/iss1/21/ (accessed February 25, 2021).
Walter, David E., and H. C. Proctor. Mites in Soil: An Interactive Key to Mites and Other Soil Microarthropods. ABRS Identification Series. Collingwood, Victoria: CSIRO Publishing, 2001.
Walters, Brianne L., John O. Whitaker, Jr., N. S. Gikas, and William J. Wrenn. “Host and Distribution Lists of Chiggers (Trombiculidae and Leeuwenhoekiidae), of North American Wild Vertebrates North of Mexico.” Faculty Publications of the Harold W. Manter Laboratory of Parasitology 697 (2011): 1–183.
Westfall, Marjorie C., Kristen K. Cecala, Steven J. Price, and Michael E. Dorcas. “Patterns of Trombiculid Mite (Hannemania dunni) Parasitism among Plethodontid Salamanders in the Western Piedmont of North Carolina.” Journal of Parasitology 94 (2008): 631–634.
Whitaker, John O., Jr., Brianne L Walters, Linda K Castor, Christopher M. Ritzi, and Nixon Wilson. “Host and Distribution Lists of Mites (Acari), Parasitic and Phoretic, in the Hair or on the Skin of North American Wild Mammals North of Mexico: Records Since 1974”. Faculty Publications of the Harold W. Manter Laboratory of Parasitology 1 (2007): 1–173.
Wicht, M. C., Jr., and A. C. Rowland. “Fauna and Distribution of Free Living Chiggers (Acarina: Trombiculidae) in Arkansas.” Proceedings of the Arkansas Academy of Science 41 (1987): 115–116. Online at http://scholarworks.uark.edu/jaas/vol41/iss1/44/ (accessed February 25, 2021).
Winter, Douglas A., Wojciech M. Zawada, and Arthur A. Johnson. “Comparison of the Symbiotic Fauna of the Family Plethodontidae in the Ouachita Mountains of Western Arkansas.” Proceedings of the Arkansas Academy of Sciences 40 (1986): 82–85. Online at http://scholarworks.uark.edu/jaas/vol40/iss1/27/ (accessed February 25, 2021).
Wolfenbarger, K. A. “Systematic and Biological Studies on North American Chiggers of the genus Trombicula, Subgenus Eutrombicula (Acarina, Trombiculidae).” Annals of the Entomological Society of America 45 (1952): 645–677.
Chris T. McAllister
Eastern Oklahoma State College
 Mites and Chiggers
Mites and Chiggers 




Comments
No comments on this entry yet.