calsfoundation@cals.org
Parasitic Crustaceans
The Subphylum Crustacea (Phylum Arthropoda) represents a diverse group of animals with members within several classes and orders, including the Amphipoda, Branchiura, Cirripedia, Copepoda, Isopoda, and Tantulocarida. (The Pentastomida, composed of parasites, are sometimes included in this subphylum and sometimes considered a separate phylum.) There are two classes with parasites: the Maxillopoda and Malacostraca. The majority of crustaceans are aquatic, living in either marine environments or fresh water, but a few groups have adapted to a terrestrial existence, such as some species of crabs and woodlice. Most crustaceans move about independently and live a free existence, although some are parasitic (about 30,000 named species) and live attached as ectoparasites to their hosts, including fish, sea, and whale lice, as well as Cymothoa exigua, the tongue-eating louse, a parasitic isopod. Among them, the parasitic copepods are dominant. Crustaceans, jointly with the monogeneans, are the most speciose group of metazoan ectoparasites of marine fishes; in addition, they infect a wide range of marine invertebrates and freshwater vertebrates. It has been estimated that three percent of all crustacean species are parasites of other Crustacea, and almost one-third of all copepods are parasitic. Thousands of species have been described, but many potential host groups are unknown; therefore, attempting to approximate estimates of species numbers is almost impossible. At present, the study of parasitic crustaceans in Arkansas is in its infancy, encompassing only a handful of reports from hosts in the state, but there are a number of species that may occur in the state but which have not yet been documented.
Evolutionarily, crustaceans are usually considered as a subphylum, and due to recent molecular analyses, it is accepted that they are paraphyletic and comprise all animals in the Pancrustacea clade other than hexapods (a subphylum that includes insects). Therefore, some crustaceans are more closely related to other hexapods and insects than they are to certain other crustaceans. Crustaceans have had an extensive fossil record, extending back to the Cambrian Period of the Paleozoic Era, and include living fossils such as the tadpole shrimp (Triops cancriformis) from Europe, the Middle East, and India, which has existed relatively unchanged for nearly 200 million years since the Upper Triassic Period. Some parasitic crustaceans such as copepods have been ectoparasites of fishes since at least the Lower Cretaceous Period, about 110 to 120 million years ago.
One of the most famous modern-day biologists working on parasitic crustaceans was Charles Branch Wilson (1861–1941), the author of numerous works on copepods. Another was Richard Rathbun (1852–1918), a Smithsonian biologist who described eight new species of copepods between 1884 and 1887. Dr. Zbigniew Kabata (1924–2014) was another whose research interest was chiefy focused on the systematics, phylogeny, and biology of parasitic copepods of fishes worldwide and the diseases caused by them. Roger F. Cressey (1930–2001), also of the Smithsonian, provided expertise on copepods parasitizing fishes. In addition to his numerous publications on these copepods, he produced important works on the Branchiura. One of the largest collections of copepods resides at the Smithsonian National Museum of Natural History (NMNH) in Washington DC.
Some common morphological features of crustaceans in general are development through a free-swimming larva called the nauplius and possession of two pairs of antennae (unlike other arthropods), one pair of mandibles, and two pairs of maxillae. Most crustaceans have gills, their heads are often indistinct and not separated well from the rest of the body, and all appendages, except sometimes for the first set of antennae (antennules), are split into two parts (biramous).
The class Maxillopoda includes species with five cephalic, six thoracic, and usually four abdominal somites plus a telson (terminal appendage) in the primitive condition; often segmentation is lost secondarily, and there are normally no appendages on the abdomen. The eye, when present in the nauplius, has a unique structure called the maxillipodan eye. The class includes five subclasses: Ostracoda, Copepoda, Brachiura, Cirripedia, and Tantulocarida; there are numerous orders. Many different taxa have parasitic members.
The subclass Ostracoda has few parasitic members, while, in general, copepods are important fish parasites of wild fishes that can have a negative effect and be a serious pest of fishery industries (particularly hatcheries) and in aquaculture. Some common adult copepod parasites of freshwater fishes are Achtheres percarum, Caligus lacustris, Ergasilus sieboldi (and other species in the genus), Lernaea cyprinacea, Salmincola californiensis, S. edwardsii, and Tracheliastes maculatus. In addition, chalimus larvae of Achtheres and Salmincola and copepodids of Lernaea attach to gill filaments and can cause epithelial hyperplasia and fusion of secondary lamellae, with consequent loss of respiratory surface area, which may be indirectly responsible for fish-kills. Attachment of copepods to the skin of fishes can also cause pressure necrosis and epidermal erosion, and the host tissue responses can include swelling, proliferation of fibroblasts, and cellular infiltration. Any lesion of the surface of a fish may also render the host susceptible to a secondary infection. Copepods also serve as intermediate hosts for important fish parasites, including cestodes (tapeworms) and nematodes (roundworms). Significant damage from these helminths may lead to fish mortalities or reduce the market value of the fish products.
One very common member of the Maxillipoda is the anchor worm, L. cyprinacea (Subclass Copepoda, Order Cyclopoida, Family Lernaeidae). It belongs to a small family that primarily infects freshwater fish. Its antennae are uniramous, the mandibles are gnathostomous (short, broad, toothed, and with an open buccal cavity), and its maxillules are biramous. Once sexually mature females are fertilized, they embed in the dermis beneath a scale near the base of fins or in the buccal cavity of freshwater fishes with an anterior holdfast organelle composed of horns derived from the cephalothorax/thorax area. The female gradually grows over a one- to two-week period and, from a small size (less than 1.5 millimeters), can grow to over 1.5 centimeters in length. Anterior holdfasts (anchors or horns) grow quite large, but legs and mouthparts are dwarfed in size by the growing female; body segmentation becomes indistinct. Egg sacs develop at the posterior end; nauplii larvae hatch and live off yolk material in their bodies through three molts and do not feed. The last nauplii molt results in a first copepodid (subadult, similar to adults prior to enlargement); these are now parasitic and seek out a fish, embed, and undergo a series of instars and molts before becoming sexually mature.
Another interesting member of the subclass Copepoda is Ergasilus spp. (Order Poecilostomatoida, Family Ergasilidae). Ergasilids are very common, and nearly all are parasitic. They are found on the skin and fins but are most frequently found on the gills of fish. They can cause significant morbidity and mortality when heavily infesting fish. They have also been implicated in promoting other fish diseases. Most species (only females) are found commonly on the gills of freshwater fishes such as eels, gars, herrings, paddlefishes, perch, pirate perch, smelts, sunfishes, temperate basses, and troutperch, although some species occur in marine (especially brackish water) fishes, such as killifish, sticklebacks, and mullets. Morphologically, the antennae of females are modified into large, sharp, claw-like structures, and the first pair of legs is modified to possess blade-like spines used for rasping off epithelial cells and mucus for consumption. There are three naupliar and five copepodid stages—all are free-living. Adult males are also free-living, and females are fertilized prior to attaching to fish. Damage to hosts may be severe gill epithelia causing loss of respiratory function, with secondary bacterial infections ensuing. Notable infections include an instance of Gizzard Shad (Dorosoma cepedianum) in Tennessee with infested skin lesions caused by E. clupeidarum. A morphologically similar taxon (Order Siphonostomatoida, Family Caligidae) to Argulus occurs in the branchial cavity of marine fishes.
Members of the subclass Branchiura include about 175 species of fish lice belonging to a single family (Argulidae) with four genera, the most important belonging to the genus Argulus. This genus contains about 129 species, has a cosmopolitan distribution, and is known from marine, estuarine, and freshwater habitats in Africa, Asia, Australia, and Europe, as well as North, Central, and South America. Thirty-two of these species and subspecies are considered valid in marine and freshwaters of the United States. They inhabit mainly freshwater habitat, both running and static water, and may occur at high density in artificial bodies of water, such as ornamental fish ponds and fish farms. Branchiuran fish lice range in length from a few to about thirty millimeters and have strongly flattened bodies, with a low profile when attached to their hosts. They are motile ectoparasites that detach from one host and swim to another. Their flat, shield-like part of the body (carapace) is fused with the head and partially covers the thorax. Two movable conspicuous compound eyes are present on the head region, and the abdomen is the tail-like part hanging off the back end. They have four pairs of swimming legs and possess two large prominent adhesive (sucking) cup-like discs derived from each of the maxillules. Just posterior to the maxillules are also large maxillae apparently used to aid in attachment. A long and slender piercing stylus is present just posterior to the antennae which the parasite uses to pierce the host skin, inject a toxin or anticoagulant, and derive nutrients. They then can use their mouth and mandibles to consume the blood, mucus, and tissue at the puncture site. Females detach from the host to lay eggs on the substrate, larvae eventually hatch, and a first-larval-stage juvenile (not a nauplius) eventually develops into an adult. Although branchiurans are primarily ectoparasites of fishes, they have occasionally been reported from the tadpoles of ranid amphibians. One species, A. japonicus, has been introduced all over the globe, primarily due to the stocking of goldfish (Carassius auratus) and carp (Cyprinus carpio). These parasites can be destructive to fishes due mainly to secondary fungal infections that attach at the puncture sites, and they can also transmit viral diseases and other fish parasites.
The class Malacostraca contains very few parasitic species and includes the orders Amphipoda, Isopoda, and Decapoda. Segmentation is distinct, and antennules of some species are biramous. Eight somites are in the thorax, with six to seven somites and a telson in the abdomen, and the first one to three thoracic appendages are modified into maxillipedes. Several members of this class can be found in North America.
Controlling certain parasitic crustaceans in fish culture has been difficult. Diflubenzuron (the active ingredient in Dimilin) is a restricted-use pesticide with a specific spectrum against crustaceans. It works by inhibiting chitin, which is necessary for forming exoskeletons, and stops molting. So, after application, it ends the life cycle of parasites like anchor worms and fish lice in about three to four days depending on the environmental temperatures. It has been especially useful in ornamental ponds, water gardens, koi ponds, and display tanks. However, it will also kill crayfish, aquatic insects like dragonflies, and other macroinvertebrates.
Study of parasitic crustaceans in Arkansas is in its infancy, as there are only a few previous reports from hosts in the state. Native fishes have been infested with Argulus spp. as follows: spotted gar (Lepisosteus oculatus) with Argulus nobilis and warmouth (Lepomis gulosus), bluegill (L. macrochirus) and largemouth bass (Micropterus salmoides) as hosts for A. mississippiensis. A. americanus infested bowfin (Amia calva) from Dorcheat Bayou in Columbia County. Lernaea catostomi has been reported from the fantail darter (Etheostoma flabellare) and L. cyprinacea, Ergasilus caeruleus, and E. celestis from various fishes. According to an Arkansas Game and Fish Commission biologist who conducted gill-netting samples near Mount Ida (Montgomery County) on Lake Ouachita, parasitic copepods, Achtheres, were observed in the mouth and gill raker area of all striped bass (Morone saxatilis) from the sample. Prevalence of the infestation was estimated be light to moderate, although it was suggested it may increase.
For additional information:
Akam, M. “Arthropods: Developmental Diversity within a (Super) Phylum.” Proceedings of the National Academy of Sciences (USA) 97 (2002): 4438–4441.
Averof, M., and M. Akam. “Insect-Crustacean Relationships: Insights from Comparative Developmental and Molecular Studies.” Philosophical Transactions of the Royal Society of London Biological Sciences 347 (1995): 293–303.
Becker, David A., W. D. Carr, Donald G. Cloutman, William A. Evans, Richard D. Heard, P. D. Holmes, M. D. Norman and W. B. Owen. Pre- and Post-Impoundment Ichthyoparasite Succession in a New Arkansas Reservoir. Arkansas Water Resources Research Center, University of Arkansas Fayetteville, Publication No. 54, 1978.
Becker, David A., and Donald G. Cloutman. “Parasites of Selected Game Fishes of Lake Fort Smith, Arkansas.” Proceedings of the Arkansas Academy of Science 29 (1975): 12–18. Online http://scholarworks.uark.edu/jaas/vol29/iss1/6/ (accessed September 25, 2019).
Boore, J. L., D. V. Lavrov, and W. M. Brown. “Gene Translocation Links Insects and Crustaceans.” Nature 392 (998): 667–668.
Bowman, T. E., and L. G. Abele. “Classification of the Recent Crustacea.” In The Biology of Crustacea, Vol. 1: Systematics, the Fossil Record, and Biogeography. Edited by L. G. Abele. New York: Academic Press, 1982.
Boxshall, G. A. B., and S. H. Halsey. An Introduction to Copepod Diversity. Hampshire, UK: The Ray Society, Intercept Ltd., 2004.
Cloutman, Donald G. “Parasite Community Structure of Largemouth Bass, Warmouth, and Bluegill in Lake Fort Smith, Arkansas.” Transactions of the American Fisheries Society 104 (1975): 277–283.
Cressey, R. F. The Genus Argulus (Crustacea: Branchiura) of the United States. Biota of Freshwater Ecosystems, U.S. Environmental Protection Agency Identification Manual 2, 1972.
Edgecombe, G. D. Arthropod Fossils and Phylogeny. New York: Columbia University Press, 1998.
Goin, Coleman J., and L. H. Ogren. “Parasitic Copepods (Argulidae) on Amphibians.” Journal of Parasitology 42 (1956): 172.
Hargis, W. J. “The Fish Parasite Argulus laticauda as a Fortuitous Human Epizoon.” Journal of Parasitology 44 (1958): 45.
Høeg, J. T. “The Biology and Life Cycle of the Cirripedia Rhizocephala.” Journal of the Marine Biology Association U.K. 75 (1995): 517–550.
Hoffman, Glenn L. Parasites of North American Freshwater Fishes. 2nd ed. Ithaca, NY: Comstock Publishing Associates, 1999.
Humes, A. G., and R. U. Gooding. “A Method for Studying the External Anatomy of Copepods.” Crustaceana 6 (1964): 238–240.
Huys, R., and G. A. Boxshall. Copepod Evolution. Ray Society Series 159. London, UK: The Ray Society, 1991.
Martin, J. W., and G. E. Davis. “An Updated Classification of the Recent Crustacea.” Natural History Museum of Los Angeles County, Science Series No. 39, 2001.
McAllister, Chris T., William J. Poly, Donald G. Cloutman, Henry W. Robison, and Michael K. Hill. “Argulus spp. (Crustacea: Branchiura) on Fishes from Arkansas and Oklahoma: New Geographic Distribution Records.” Proceedings of the Oklahoma Academy of Science 70 (2016): 70–72.
Meehean, O. L. “A Review of the Parasitic Crustacea of the Genus Argulus in the Collections of the United States National Museum.” Proceedings of the United States National Museum 88 (1940): 459–522.
Overstreet, Robin M., Iva Dyková, and W. E. Hawkins. “Branchiura.” In Microscopic Anatomy of the Invertebrates, Vol. 9: Crustacea. Edited by F. W. Harrison and A. G. Humes. New York: Wiley-Liss, Inc., 1992.
Piasecki, Wojciech, Andrew E. Goodwin, Jorge C. Eiras, and Barbara F. Nowak. “Importance of Copepoda in Freshwater Aquaculture.” Zoological Studies 43 (2004):193–205.
Poly, William J. “Global Diversity of Fishlice (Crustacea: Brachiura: Argulidae) in Freshwater.” Hydrobiologia 595 (2008): 209–212.
Regier, J. C., and J. W. Shultz. “Molecular Phylogeny of the Major Arthropod Groups Indicates Polyphyly of Crustaceans and a New Hypothesis for the Origin of Hexapods.” Molecular Biology and Evolution 14 (1997): 902–913.
Roberts, Larry S. “Ergasilus (Copepoda: Cyclopoida): Revision and Key to Species in North America.” Transactions of the American Microscopical Society 89 (1970): 134–161.
Roberts, Larry S., and John Janovy Jr. Foundations of Parasitology. 9th ed. Boston: McGraw-Hill Higher Education, 2012.
Rogers, William A., and J. P. Hawke. “The Parasitic Copepod Ergasilus from the Skin of the Gizzard Shad Dorosoma cepedianum.” Transactions of the American Microscopical Society 97 (1978): 244.
Shields, Jeffrey D., and Christopher B. Boyko. “Introduction to the First Symposium on the Biology of the Parasitic Crustacea.” Integrative and Comparative Biology 49 (2009): 93–94.
Yamaguti, Satyu. Parasitic Copepoda and Branchiura of Fishes. New York: Wiley Interscience Publishers, 1963.
Chris T. McAllister
Eastern Oklahoma State College
Henry W. Robison
Sherwood, Arkansas
 Science and Technology
Science and Technology Lernaea cyprinacea
Lernaea cyprinacea 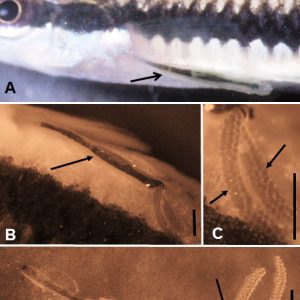 Lernaea cyprinacea
Lernaea cyprinacea 




The subphylum Crustacea as a whole is not parasitic. There are a few parasitically living taxa within the subphylum Crustacea. Also, most recent phylogeny would not recognize a subphylum Crustacea anymore, but that is difficult to keep track of.