calsfoundation@cals.org
Myxozoans
Myxozoans are a group of microscopic, oligocellular, obligate endoparasites that belong to the Phylum Cnidaria, which also includes sea anemones, box jellies, corals, true jellies, sea pens, and hydrozoans. There are two parasitic classes, the Malacosporea and Myxosporea, and more than 2,200 nominal species of myxozoans classified into sixty-four genera and seventeen families. Myxozoans were, for years, placed within their own phylum (Myxozoa). Similarities to cnidarians had been noted at various times but not firmly until 2007. Although morphological and genetic evidence support placement of the Myxozoa as cnidarians, and this taxonomy has been followed by some authorities, others have not reached the same conclusion; exactly where the Myxozoa fit in this taxonomic scheme is not yet entirely known. Less is known about the myxozoans that are found in Arkansas hosts compared with other states. Several species, however, have been reported from the state in fishes and amphibians; however, Myxozoans do not appear to be a severe threat to Arkansas’s fisheries.
Myxozoans are parasites of aquatic poikilothermic (“cold-blooded”) vertebrates and invertebrates (annelids and oligochaetes) but most commonly encountered in wild and cultured freshwater teleost (bony fishes) and marine fishes of the world. Indeed, more than 2,200 species have been described from fish alone. In addition, there have been three species reported from agnathans (hagfishes and lampreys), thirty-five species in cartilaginous fishes (Chondrichthyes), and the remainder in bony fishes. Approximately thirteen species have been reported from amphibians and about six in chelonian reptiles (turtles). A few species have been documented to occur in the myxospore phase in invertebrates, including a species of Kudoa in a giant octopus, Enteroctopus dofleini. Myxozoans inhabit a wide range of the host anatomy, including bone, brain, cartilage, connective tissue, eyes, fins, heart, liver, mesenteries, peritoneum, muscle, scales, spinal cord, spleen, swim bladder, viscera, gills, skin, intestinal tract, kidney, gallbladder, gonads, urinary bladder, and other miscellaneous sites.
The Class Malacosporea has only three species with unhardened (soft) shell valves, including those in the genera Buddenbrockia and Tetracapsuloides, parasites of bryozoans and fish. Their main diagnostic characteristics are possession of worm-like (myxoworms or vermiform) bodies and sac-like trophic (proliferative) stages. Unfortunately, developing malacospores (mature spores) do not possess distinctive diagnostic taxonomic characters for classification. Usage of the few morphological characters as well as molecular (DNA sequence) data is necessary to help reveal diversity in this class. Buddenbrockia plumatellae was described from the coelomic cavity of the fungoid bryozoan Plumatella fungosa in Belgium but is now cosmopolitan in distribution. Tetracapsuloides bryosalmonae was originally reported from three species of bryozoans from Michigan and Ohio. It is the agent (“PKX”) of proliferative kidney disease of wild and cultured salmonid fishes and pike in North America and Europe. Mortalities in fish ranges from thirty to fifty percent, particularly in fry.
The larger Class Myxosporea includes several major genera of myxozoans having spores with hardened shell valves occurring in fishes and amphibians. Examples include Chloromyxum, Cystodiscus, Myxidium, Henneguya, Kudoa, Myxobolus, and Sphaerospora. Some representative North American species are Chloromyxum trijugum in the gallbladder of centrarchid fishes, Henneguya exilis in the gill filaments of channel catfish (Ictalurus punctatus), Cystodiscus serotinus in the gallbladder of frogs and toads, and Myxobilatus mictospora in the urinary bladder of largemouth bass (Micropterus salmoides). In Europe, the following species are found in fishes and include Myxobolus cyprini in muscles of carp; Myxobolus cerebralis (first reported in Germany in the late 1890s), which was introduced along with European Brown Trout (Salmo trutta) into North America, infecting fish cartilage and causing deformities in trout, salmon, and their allies (including wild fish populations from cooler waters); Sphaerospora renicola in the renal tubuli of carp; and Thelohanellus nikolskii in cysts on the fins of carp.
Known myxozoan life cycles describe sporogony in a vertebrate host producing myxospores. These infect an oligochaete or polychaete (both annelid worms) in which a different type of sporogony produces actinospores. In a general life cycle, following extrusion of the polar filaments and sporoplasm release, the sporoplasm penetrates the gut wall and develops into a multinucleate trophozoite (amoeboid plasmodium, a large cytoplasmic mass with many nuclei) in the appropriate tissues or in the lumen. The plasmodium is formed by an amoeboid cytoplasm that eventually increases in size, and the nucleus undergoes repeated karyokinesis. Plasmodia grow attached to the epithelium in the coelomic area in coelozoic forms (i.e., urinary bladder, swim bladder), or occur within tissues themselves in histozoic forms. Infective spores are produced that are transmitted to the next host. Each spore contains one to several (usually two) nematocyst-like polar capsules each with a coiled polar filament that is expelled during invasion and one or more sporoplasms. Each spore comprises one to seven spore shell valves. Spores have traditionally been thought to require aging in water before they become infective; however, recent reports have shown that, at least with M. cerebralis in salmonid fishes, tubificid worms are required as intermediate hosts in its life cycle. This may explain why seemingly fully developed spores usually do not transmit the infection directly to the definitive host.
Some myxozoans possess cells that are destined to form spores within the plasmodia. The following represents spore formation in Henneguya exilis in I. punctatus. An envelope cell encapsulates a sporogonic cell and, following multiple cell divisions of the sporogonic cell, forms ten new cells that eventually form two separate spores within the envelope cell. These two spores from the same cells are termed “disporoblastic spore formation.” Other types of spore formation occur in myxozoans as follows: four capsulogenic cells (two/spore, one/polar capsule); four valvulogenic cells (two/spore, one/each valve); and two sporoplasms (one/spore).
Myxobolus cerebralis is an economically important myxozoan that received relatively little attention until the discovery of its two-host life cycle in the mid-1980s. Interestingly, it was also the first complete myxozoan life cycle to be described. However, in the last several decades, there has been a renewed interest in this parasite because whirling disease has been implicated in the decline of wild trout populations in several western U.S. states. Subsequent research efforts have dramatically increased our understanding of the biology of M. cerebralis and the numerous factors that affect the severity of whirling disease in salmonid hosts. These efforts also have provided a great deal of new information concerning interactions between M. cerebralis and its aquatic oligochaete host. Indeed, the parasite is also readily transferred unknowingly by birds and humans into uncontaminated waters facilitating further infection of sport fishes. In turn, anglers are affected the most by the reduction of the sportfish. Unfortunately, overall ecological balance of streams and biodiversity are also affected by the loss of trout and salmon.
In the life cycle of M. cerebralis, spores are liberated into the aqueous environment where they are ingested by a definitive host tubificid oligochaete (Tubifex tubifex). Interestingly, spores are able to survive twenty to thirty years in a stream before finding the intermediate host. The parasite is also able to survive freezing temperatures and desiccation in the spore stage. Polar filaments are expelled, valves separate, and the sporoplasm invades its gut (usually, intracellular spaces between intestinal epithelial cells).
The sporoplasm divides asexually, sometimes by multiple fission; gamogony occurs, where cells derived from different plasmodia fuse; and triactinomyxon (three-valve) spores released into the lumen of the gut. Spores in the water attach to salmonid fishes (reservoir hosts) via polar filaments, and sporoplasms invade the cells of the primary host. Fish can also become infected by ingesting infected oligochaetes. The head cartilage of fishes is penetrated followed by rapid reproduction of the parasite. As the parasite population increases, so does the pressure applied to the equilibrium organ of the host to cause it to swim in characteristic constant circles or whirls. This prevents the host fish from foraging for food and evading predators, ultimately resulting in its death. Whirling disease has a devastating effect on many popular sport fish by drastically reducing populations.
Another important myxosporean pathogen of fishes, Ceratonova (=Ceratomyxa) shasta is a parasite that infects salmonid fishes on the Pacific coast of North America. It was first observed around 1950 at the Crystal Lake Hatchery in Shasta County, California, and has now been reported from other sites in the Pacific Northwest, including those in Alaska, Idaho, Oregon, Washington, and British Columbia (Canada). In addition to the fish host, C. shasta infects a freshwater polychaete worm. In its life cycle, actinospores are released from the polychaete and infect fish on contact in the water column. Spores are released back into freshwater system after its fish host dies; however, the complete life cycle and hosts are not fully understood (especially the ecology of the polychaete worm). Pathologies in salmonid fishes from infections of C. shasta include lethargy, weight loss, skin darkening, formation of ascites, exopthalmia of the eyes, and pustules in the kidneys. However, these symptoms can vary from one salmonid species to another, and also depend on life stage of the host. Interestingly, infection in adult Chinook salmon (Oncorhynchus tshawytscha) causes death through perforation of the intestinal wall with concomitant bacterial infection. Progression of the infection and mortality is temperature dependent, with higher temperature increasing disease progression and resulting in rapid mortality.
Members of the myxozoan genus Kudoa primarily infect the skeletal muscle of marine fishes, where the parasite forms nodules or pseudocysts in the host, containing huge numbers of individual spores. In major infections, severe inflammation surrounds infected muscle fibers and leads to post-mortem degeneration of the tissue, or soft-flesh disease. In fisheries and in the aquaculture industries, losses to salmon occur post-harvest because the fish appear normal upon capture, but protein-degrading enzymes released by the parasite decrease the quality of the muscle.
Compared to other states, knowledge of the myxozoans that are found in Arkansas hosts warrants additional study. Several species, however, have been reported from the state in fishes including Henneguya adiposa and H. exilis in I. punctatus, Myxobolus argenteus and M. notemigoni in golden shiners (Notemigonus crysoleucus), and M. microcystis in largemouth bass (Micropterus salmoides). In Arkansas amphibians, myxozoans have been documented in three salamanders, three species of frogs, and a single species of toad. A new species of Chloromyxum (C. salamandrae) was reported from the gallbladder epithelium of many-ribbed salamanders (Eurycea multiplicata) from Saline County, in Oklahoma salamanders (Eurycea tynerensis) from Conway and Van Buren counties, and from Ouachita dusky salamanders (Desmognathus brimleyorum) from Polk County. Cystodiscus spp. has been found in the gallbladder of pickerel frogs (Rana palustris) from Independence County, wood frogs (Rana sylvatica) from Izard County, and dwarf American toads (Anaxyrus americanus charlesmithi) and Cajun chorus frogs (Pseudacris fouquettei), both originating from Union County.
Myxozoans do not appear to be a particular threat to Arkansas’s fisheries. However, not enough is known about their effect on fish in the state, and additional studies are sorely needed. To date, whirling disease has not been reported from any cultured, wild, or introduced fish of the state, particularly different species of trout. The pathogen (M. cerebralis) can be managed if fish coming into the state are cultured in spore-free source water, in concrete raceways with strong water flows, or in ponds that are regularly disinfected. Constant monitoring for the presence of spores can also help in managing the pathogen.
Suggestions for future studies include addressing continuing taxonomic and phylogenetic inconsistencies. In addition, proposals should be made for describing taxa in the absence of molecular data and/or when DNA sequence and morphological data are dissimilar. Several potential hosts remain to be examined for myxozoans in Arkansas, and once they are, new host and geographic records, including the discovery of new species are distinct possibilities.
For additional information:
Barnard, Susan M., and Steve J. Upton. A Veterinary Guide to the Parasites of Reptiles. Vol. 1: Reptiles. Malabar, FL: Kreiger Publishing Company, 1994.
Bartholomew, J. L. “Host Resistance to Infection by the Myxosporean Parasite Ceratomyxa shasta: A Review.” Journal of Aquatic Animal Health 10 (1998): 112–120.
Canning, Elizabeth U., and Beth Okamura. “Biodiversity and Evolution of the Myxozoa.” Advances in Parasitology 56 (2004): 44–131.
Current, William L., and John Janovy Jr. “Sporogenesis in Henneguya exilis Infecting the Channel Catfish: An Ultrastructural Study.” Protistologica 13 (1977): 157–167.
Densmore, C. L., V. S. Blazer, T. B. Waldrop, and P. S. Pooler. “Effects of Whirling Disease on Selected Hematological Patterns in Rainbow Trout.” Journal of Wildlife Diseases 37 (2001): 375–378.
Eiras, Jorge C. “Synopsis of the Species of the Genus Henneguya Thélohan, 1892 (Myxozoa: Myxosporea: Myxobolidae).” Systematic Parasitology 52 (2002): 43–54.
Eiras, Jorge C., K. Molnár and Y. S. Lu. “Synopsis of the Species of Myxobolus Butschli, 1882 (Myxozoa: Myxosporea: Myxobolidae).” Systematic Parasitology 61 (2005): 1–46.
Eiras, Jorge C., A. Saraiva, C. F. Cruz, M. J. Santos, and Ivan Fiala. “Synopsis of the Species of Myxidium Bütschli, 1882 (Myxozoa: Myxosporea: Bivalvulida).” Systematic Parasitology 80 (2011): 81–116.
Fiala, Ivan, Pavla Bartošová-Sojková, and Christopher M. Whipps. Classification and Phylogenetics of Myxozoa. In Myxozoan Evolution, Ecology and Development, edited by B. Okamura et al. Switzerland: Springer International Publishing, 2015.
Foox, Jonathan, and Mark E. Siddall. “The Road to Cnidaria: History of Phylogeny of the Myxozoa.” Journal of Parasitology 101 (2015): 269–274.
Gilbert, M. A., and William O. Granath Jr. “Whirling Disease of Salmonid Fish: Life Cycle, Biology, and Disease.” Journal of Parasitology 89 (2003): 658–667.
Gunter, N. L., Christopher M. Whipps, and R. D. Adlard. “Ceratomyxa (Myxozoa: Bivalvulida): Robust Taxon or Genus of Convenience?” International Journal for Parasitology 39 (2009): 1395–1405.
Gurley, R. R. “On the Classification of the Myxosporidia, a Group of Protozoan Parasites Infesting Fishes.” Bulletin of the United States Fisheries Commission 11 (1893): 407–420.
Gurley, R. R. “The Myxosporidia, or Psorosperms of Fishes, and the Epidemics Produced by Them.” Bulletin of the United States Fisheries Commission 26 (1894): 65–304.
Hartdigan, Ashlie, Ivan Fiala, Iva Dyková, Karrie Rose, David N. Phalen, and Jan Šlapeta. “New Species of Myxosporea from Frogs and Resurrection of the Genus Cystodiscus Lutz, 1889 for Species with Myxospores in Gallbladders of Amphibians.” Parasitology 139 (2012): 478–496.
Hoffman, Glenn L. Parasites of North American Freshwater Fishes. 2nd ed. Ithaca, NY: Comstock Publishing Associates, Cornell University Press, 1999.
Hoffman, Glenn L., and R. E. Putz. “Effect of Freezing and Aging on the Spores of Myxobolus cerebralis, the Causative Agent of Salmonid Whirling Disease.” Progressive Fish Culturist 33 (1971): 95–98.
Kodádková, A., Iva Dyková, Tomáš Tyml, Oleg Ditrich, and Ivan Fiala. “Myxozoa in High Arctic: Survey on the Central Part of Svalbard Archipelago.” International Journal for Parasitology: Parasites and Wildlife 3 (2014): 41–56.
Kudo, Richard R. “Studies on Myxosporidia. A Synopsis on Genera and Species of Myxosporidia.” Illinois Biological Monographs 5 (1919): 1–265.
———. “A Taxonomic Consideration of Myxosporidia.” Transactions of the American Microscopical Society 52 (1933): 195–216.
———. “Further Observations on the Protozoan, Myxidium serotinum, Inhabiting the Gall Bladder of North American Salientia.” Journal of Morphology 72 (1943): 263–277.
———. Protozoology. 5th ed. Springfield, IL: Charles C. Thomas Publishers, 1966.
Landsberg, J. H., and Jirí Lom. “Taxonomy of the Genus Myxobolus (Myxobolidae, Myxosporea): Current Listing of Species and Revision of Synonyms.” Systematic Parasitology 18 (1991): 165–168.
Lom, Jirí. “Phylum Myxozoa.” In Handbook of Protoctista, edited by Lynn Margulis, John O. Corliss, M. Melkonian, and D. J. Chapman. Boston: Jones and Bartlett, 1990.
Lom, Jirí, and Iva Dyková. “Myxozoan Genera: Definition and Notes on Taxonomy, Life-Cycle, Terminology and Pathogenic Species” Folia Parasitologica 53 (2006): 1–36.
Lom, Jirí, and Elmer R. Noble. “Revised Classification of the Myxosporea Buetschli, 1881.” Folia Parasitologica 31 (1984): 193–205.
Markiw, M. E., and K. Wolf. “Myxosoma cerebralis (Myxozoa: Myxosporea) Etiologic Agent of Salmonid Whirling Disease Requires Tubificid Worm (Annelida: Oligochaeta) in its Life Cycle.” Journal of Protozoology 30 (1993): 561–564.
McAllister, Chris T., Charles R. Bursey, and Dana M. Calhoun. “Symbiotic Protista and Helminth Parasites of the Cajun Chorus Frog, Pseudacris fouquettei (Anura: Hylidae), from Oklahoma.” Proceedings of the Oklahoma Academy of Science 95 (2015): 83–92.
McAllister, Chris T., Charles R. Bursey and Matthew B. Connior. “Helminth Parasites of the Dwarf American toad, Anaxyrus americanus charlesmithi (Anura: Bufonidae), from Arkansas and Oklahoma.” Proceedings of the Oklahoma Academy of Science 94 (2014): 51–58.
McAllister, Chris T., Charles R. Bursey, Matthew B. Connior, Stanley E. Trauth. “Symbiotic Protozoa and Helminth Parasites of the Cajun Chorus Frog, Pseudacris fouquettei (Anura: Hylidae), from Southern Arkansas and Northeastern Texas, U.S.A.” Comparative Parasitology 80 (2013): 96–104.
McAllister, Chris T., Charles R. Bursey, and Stanley E. Trauth. “Parasites of the Pickerel Frog, Rana palustris (Anura: Ranidae), from the Southern Part of its Range.” Southwestern Naturalist 40 (1995): 111–116.
McAllister, Chris T., Charles R. Bursey, and Stanley E. Trauth. “New Host and Geographic Distribution Records for Some Endoparasites (Myxosporea, Trematoda, Cestoidea, Nematoda) of Amphibians and Reptiles from Arkansas and Texas, U.S.A.” Comparative Parasitology 75 (2008): 241–254.
McAllister, Chris T., Charles R. Bursey, Steve J. Upton, Stanley E. Trauth, and David B. Conn. “Parasites of Desmognathus brimleyorum (Caudata: Plethodontidae) from the Ouachita Mountains of Arkansas and Oklahoma.” Journal of the Helminthological Society of Washington 62 (1995): 150–156.
McAllister, Chris T., and Stanley E. Trauth. “New Host Records for Myxidium serotinum (Protozoa: Myxosporea) from North American Amphibians.” Journal of Parasitology 81 (1995): 485–488.
McAllister, Chris T., Stanley E. Trauth and Ben L. J. Delvinquier. “Ultrastructural Observations on Myxidium serotinum (Protozoa: Myxosporea) from Bufo speciosus (Anura: Bufonidae) in Texas.” Journal of the Helminthological Society of Washington 62 (1995): 229–232.
McAllister, Chris T., Steve J. Upton, Stanley E. Trauth, and Charles R. Bursey. “Parasites of Wood Frogs, Rana sylvatica from Arkansas, with a Description of a New Species of Eimeria (Apicomplexa: Eimeriidae).” Journal of the Helminthological Society of Washington 62 (1995): 143–149.
Moran, J. D. W., D. J. Whitaker, and Michael L. Kent. “A Review of Myxosporean Genus Kudoa Meglitsch, 1947, and its Impact on the International Aquaculture Industry and Commercial Fisheries.” Aquaculture 172 (1990): 163–196.
Noble, Elmer R. “On a Myxosporidian (Protozoan) Parasite of California Trout.” Journal of Parasitology 36 (1950): 457–460.
Okamura, Beth, Alexander Gruhl, and Jerri L. Bartholomew, eds. Myxozoan Evolution, Ecology and Development. New York: Springer Publishers, 2015.
Pote, Linda M., L. A. Hanson, and R. Shvaji. “Small Subunit Ribosomal RNA Sequences Link the Cause of Proliferative Gill Disease in Channel Catfish to Henneguya ictaluri (Myxozoa: Myxosporea).” Journal of Aquatic Animal Health 12 (2000): 230–240.
Roberts, Larry S., and John Janovy Jr. Foundations of Parasitology. 9th ed. Boston: McGraw-Hill Higher Education, 2012.
Siddall, Mark, D. S. Martin, D. Bridge, Sherwin S. Desser, and David K. Cone. “The Demise of a Phylum of Protists: Phylogeny of Myxozoa and Other Parasitic Cnidaria.” Journal of Parasitology 81 (1995): 961–967.
Sitja-Bobadilla, Ariadna, and Pilar Alvarez-Pellitero. “Revised Classification and Key to Species of the Genus Sphaerospora Davies, 1917 (Protozoa, Myxosporea).” Research and Reviews in Parasitology 54 (1994): 67–80.
Smothers, J., C. von Dohlen, L. Smith, and R. Spall. “Molecular Evidence that the Myxozoan Protists are Metazoans.” Science 265 (1994): 1719–1721.
Upton, Steve J., Chris T. McAllister, and Stanley E. Trauth. “A New Species of Chloromyxum (Myxozoa: Chloromyxidae) from the Gall Bladder of Eurycea spp. (Caudata: Plethodontidae) in North America.” Journal of Wildlife Disease 31 (1995): 394–396.
Whipps, Christopher M., John W. Fournie, D. A. Morrison, C. Azevedo, E. Matos, P. Thebo, and Michael L. Kent. “Phylogeny of Fish-infecting Calyptospora Species (Apicomplexa: Eimeriorina).” Parasitology Research 111 (2012): 1331–1342.
Whipps, Christopher M., G. Grossel, R. D. Adlard, H. Yokoyama, M. S. Bryant, B. L. Munday, and Michael L. Kent. “Phylogeny of the Multivalvulidae (Myxozoa: Myxosporea) Based Upon Comparative rDNA Sequence Analysis.” Journal of Parasitology 90 (2004): 618–622.
Whipps, Christopher M., and Y. Zhao. “Synopsis of the Species of the Genus Sphaeromyxa Thélohan, 1892 (Myxosporea: Bivalvulida: Variisporina: Sphaeromyxidae).” Systematic Parasitology 92 (2015): 81–99.
Winnipennickx, B., Y. Van de Peer, and T. Backeliau. “Metazoan Relationships on the Basis of 18S rRNA Sequences: A Few Years Later.” American Zoologist 38 (1998): 888–906.
Chris T. McAllister
Eastern Oklahoma State College
 Esocids
Esocids Science and Technology
Science and Technology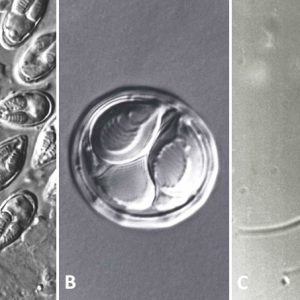 Myxozoan Spores
Myxozoan Spores 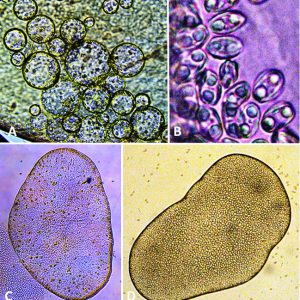 Myxozoans
Myxozoans  Whirling Disease
Whirling Disease 




Comments
No comments on this entry yet.